Preparation method of low melting point drug solid dispersion
A technology of solid dispersion and low melting point, which can be applied to medical preparations containing non-active ingredients, medical preparations containing active ingredients, and drug delivery, etc. Bioavailability, environmental friendliness, effect of reducing operation steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
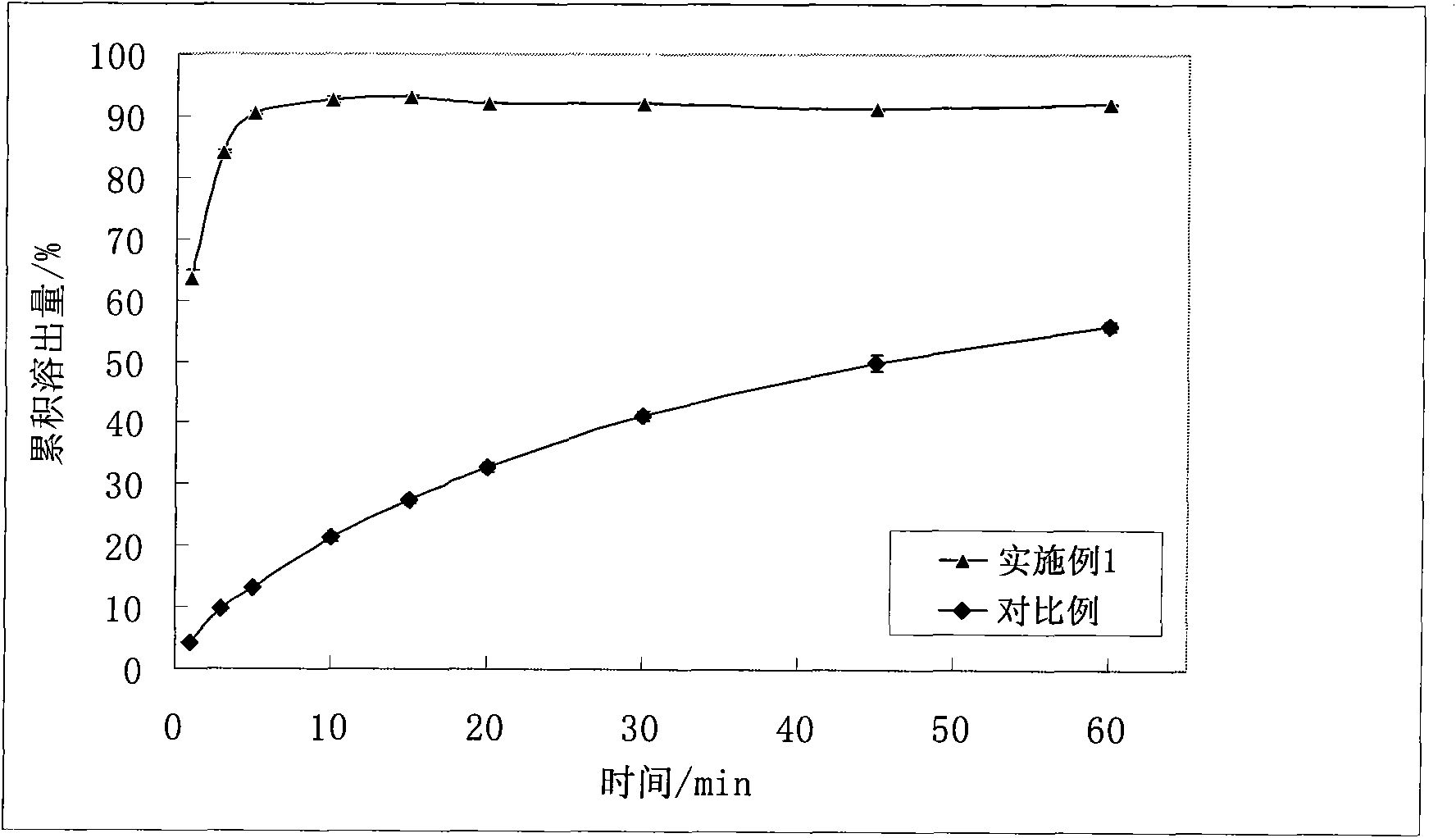
Image
Examples
Embodiment 1
[0024] α-Asarone 30g
[0025] Low-substituted hydroxypropyl cellulose (LH-21) 236.4g
[0026] Sodium carboxymethyl starch 24g
[0028] Magnesium Stearate 1.5g
[0029] Micronized silica gel 0.6g
[0030] Sodium Lauryl Sulfate 4.5g
[0031] Prepare the raw material α-asarone and each auxiliary material according to the above prescription and pass through an 80-mesh sieve, mix evenly, seal, keep the temperature at 65°C for 1.5h, cool to room temperature, and obtain a solid dispersion, lightly crush and pass through a 40-mesh sieve Sieve, and compress into 1000 tablets according to the weight of each tablet of 0.3g, to obtain immediate-release α-asarone tablets.
Embodiment 2
[0033] Ibuprofen 100g
[0034] Microcrystalline Cellulose (PH101) 135.5g
[0035] Sodium carboxymethyl starch 12g
[0037] Magnesium stearate 0.7g
[0038] Micronized silica gel 0.3g
[0039] The raw material ibuprofen and various auxiliary materials were prepared according to the above prescription and passed through an 80 mesh sieve, mixed evenly, sealed, kept at a constant temperature of 85°C for 2 hours, cooled to room temperature to obtain a solid dispersion, lightly crushed, passed through a 40 mesh sieve, and Press each tablet weight 0.25g and press into 1000, obtain quick-release ibuprofen tablet.
Embodiment 3
[0041] Paeonol 40g
[0042] Low-substituted hydroxypropyl cellulose (LH-21) 229.4g
[0043] Sodium carboxymethyl starch 24g
[0045] Magnesium Stearate 1.5g
[0046] Micronized silica gel 0.6g
[0047] Sodium Lauryl Sulfate 1.5g
[0048] Prepare the raw materials and various auxiliary materials according to the above prescription and pass through a 80-mesh sieve, mix evenly, seal, keep the temperature at 60°C for 1.5h, cool to room temperature, and after obtaining a solid dispersion, lightly press and crush, pass through a 40-mesh sieve, and press Each tablet weighing 0.3 g was pressed into 1000 tablets to obtain immediate-release paeonol tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com