Method for detecting necrosis degree of hepatic cells through level of plasma free DNA
A liver cell necrosis and level detection technology, which is applied in the field of plasma free DNA level detection of liver cell necrosis, can solve the problems of other researches that have not been reported in the literature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
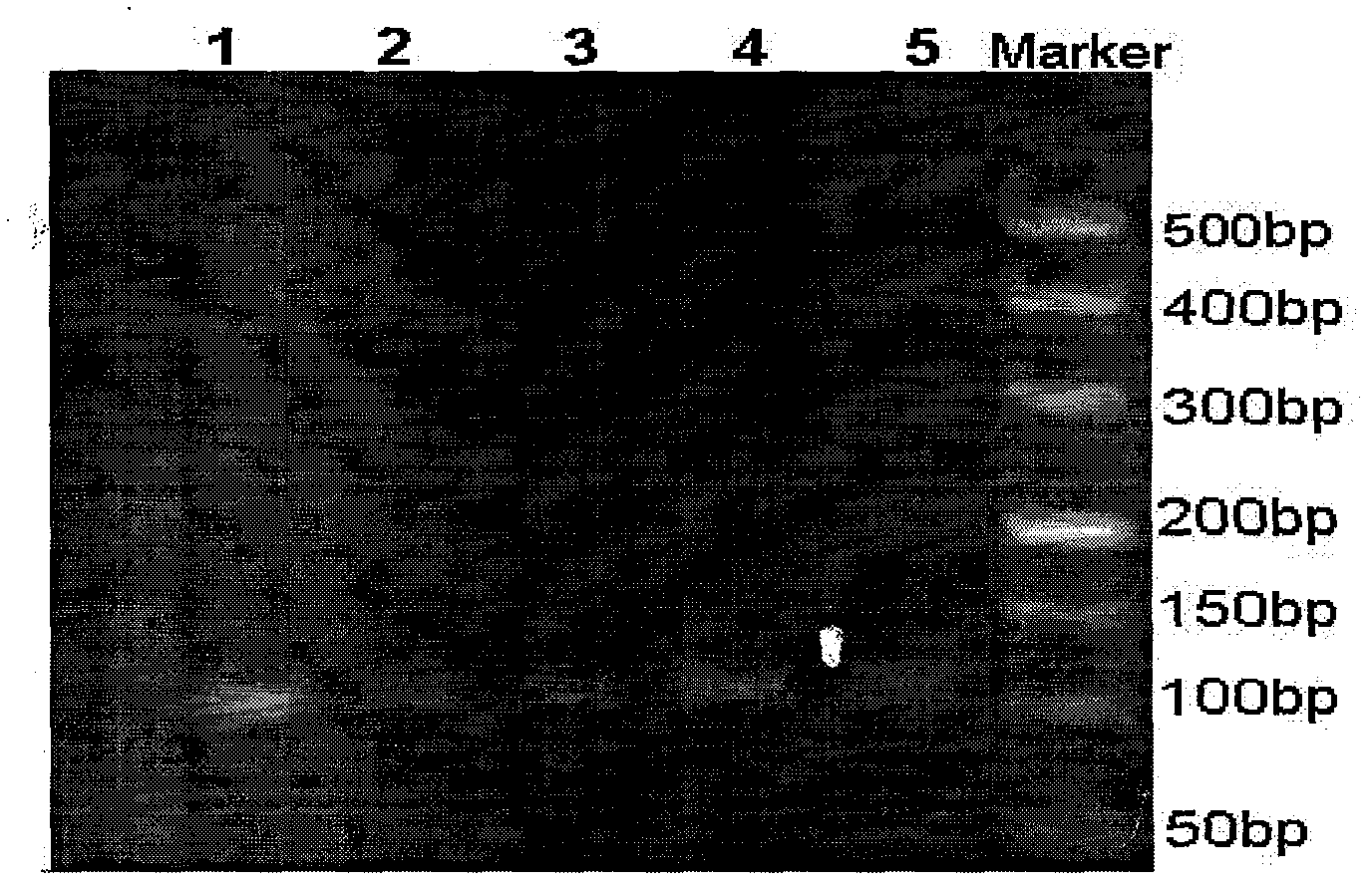
[0026] Example 1, preparation of primer β-globin standard.
[0027] 1. Main reagents and equipment for the experiment
[0028] 1) Commonly used chemical reagents such as sodium hydroxide, potassium hydroxide, sodium chloride, potassium chloride, absolute ethanol, glacial acetic acid, ammonium acetate, sodium acetate, and isoamyl alcohol are all produced by Xi’an Chemical Reagent Factory, and the purity level is analytical pure;
[0029] 2) EB (subpackaged by Sigma Company, purchased from Huamei Bioengineering Company);
[0030] 3) loading buffer (TaKaRa products of Treasure Biotechnology, purchased from Xi'an Zhou Dingguo Bioengineering Company);
[0031] 4) agar powder (GEGE TECH Shanghai Co., Ltd., purchased from Xi'an Zhou Dingguo Biological Engineering Company);
[0032] 5) Agarose (the original product of Amresco Company, purchased from Xi'an Zhou Dingguo Bioengineering Company);
[0033] 6) Nucleic acid molecular weight reference material 500bp DNA maker, 2000bp DNA ...
Embodiment 2
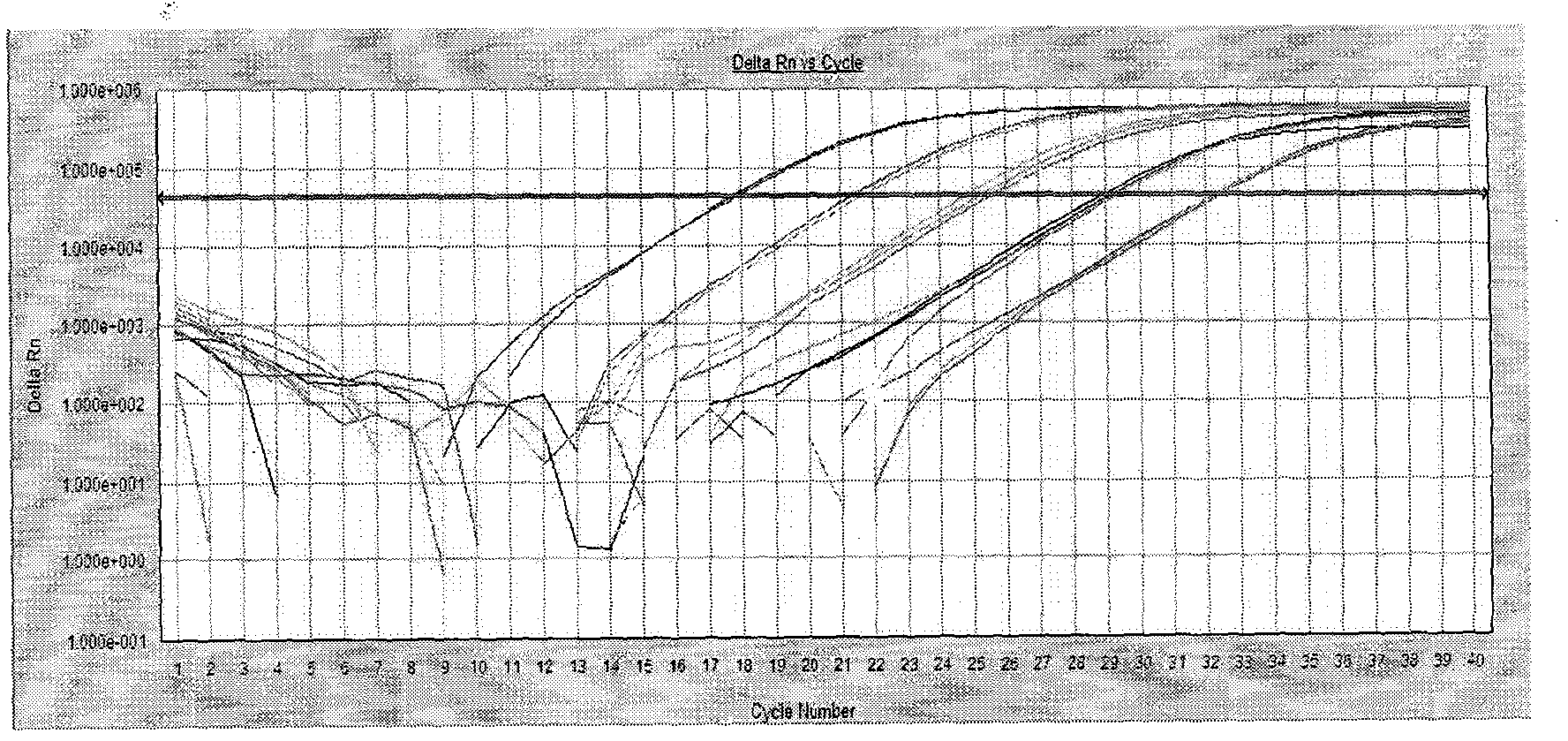
[0155] Embodiment two, extraction and quantification of plasma nucleosome
[0156] 1. The main materials of the experiment
[0157] Fluorescent PCR reaction mix SYBR Premix Ex Taq TM , with SYBR Green I, reaction buffer, dNTP, TaKaRa Ex Taq TM HS, magnesium ions (TaKaRa product of Bao Biotechnology, purchased from Bao Tech Bioengineering Company), and reagents were stored according to the instructions.
[0158]
[0159] 2. Research object
[0160] A total of 204 patients with acute and chronic liver damage and liver cancer caused by hepatitis B, hepatitis C, and drug-induced hepatitis were admitted to the Department of Infectious Diseases of the First Affiliated Hospital of Xi'an Jiaotong University School of Medicine from March 2009 to June 2010. The clinical diagnosis was in line with the viral hepatitis prevention and treatment program revised by the Xi'an Conference in 2000. Aged 5-79 years old, with an average of 45.14±14.547 years old; 146 males, aged 5-79 year...
Embodiment 3
[0192] Embodiment three, clinical experiment
[0193] 1. Research object
[0194]A total of 204 patients with hepatitis B virus, hepatitis C virus and drug-induced acute and chronic liver damage and liver cancer were admitted to the Department of Infectious Diseases of the First Affiliated Hospital of Xi'an Jiaotong University School of Medicine from March 2009 to June 2010 , to rule out various immune diseases. There is no absolute pathological boundary between clinical diagnosis of chronic hepatitis and compensated liver cirrhosis. Some clinical diagnoses of chronic hepatitis may have shown early liver cirrhosis, both of which are mainly inflammatory reactions; severe hepatitis, acute hepatitis and liver cancer all present Necrosis or apoptosis of liver cells or cancer cells, mainly cell death. The patients were divided into three groups: the first group was CH and LC group, including patients with chronic hepatitis and liver cirrhosis; the second group was DLC group, incl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com