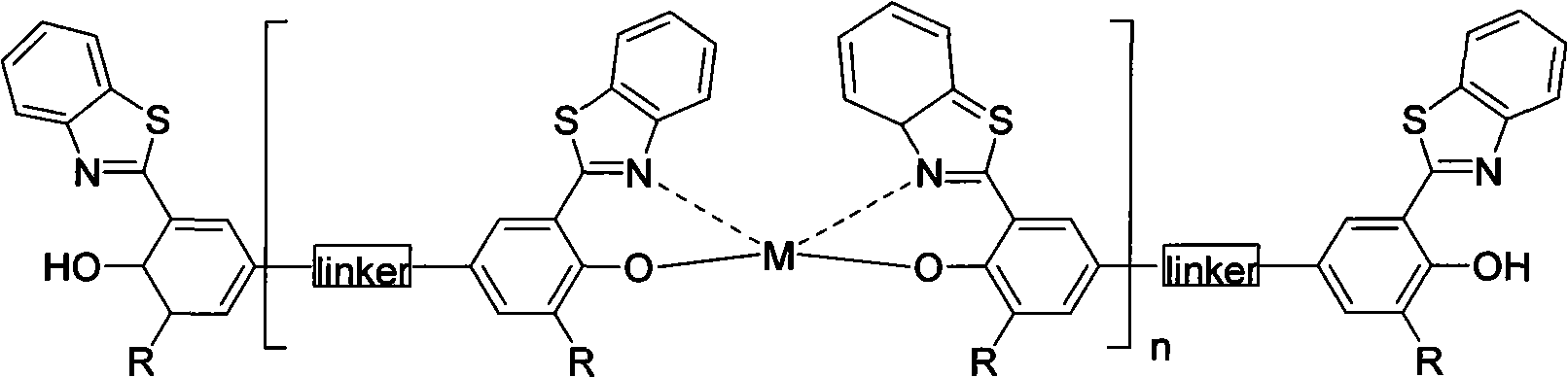
Benzothiazole derivatives metal coordination polymer based on bridged bis-salicylaldehyde structure as well as manufacture method and application thereof
A technology of benzothiazoles and metal coordination, which is applied in the synthesis of metal coordination polymers, the synthesis of benzothiazole ligands, and the application fields of organic electroluminescent materials to achieve good thermal stability and process operation Simple, solubility-improving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032]
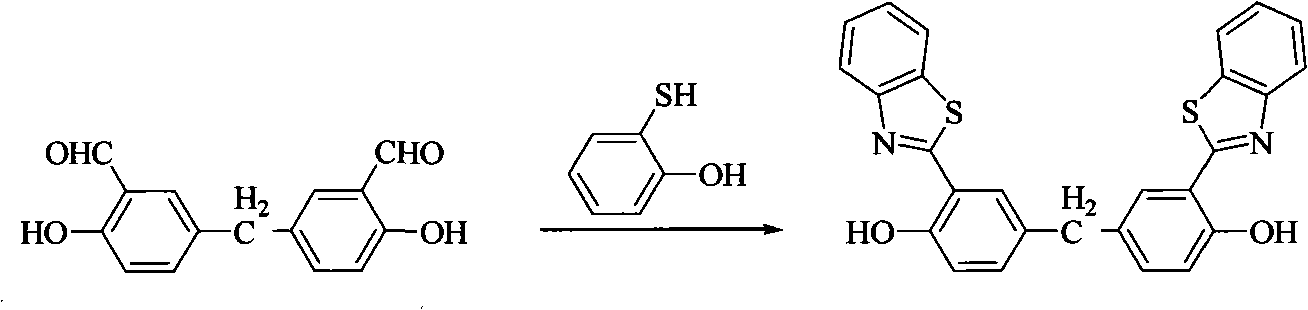
[0033] Add 128mg (0.5mmol) of aldehyde, 131mg (1.05mmol, 2.1eq) of aminothiophenol, 2.5mL of DMSO into the sealed tube, and react at 185°C for 3h. After the reaction solution was cooled to room temperature, it was poured into 60mL water to form a white emulsion, and 5mL saturated saline was added to break the emulsion, CH 2 Cl 2 Extraction (15 mL x 3). The organic phases were combined, dried and concentrated, followed by column chromatography (petroleum ether / ethyl acetate=20:1) to obtain a pale yellow solid, which was the ligand, with a yield of 36%. 1 H NMR (CDCl 3 , 300MHz, ppm) δ: 12.45(s, 2H), 7.99(d, J=8.1Hz, 2H), 7.89(d, J=7.8Hz, 2H), 7.53~7.48(m, 4H), 7.41(t , J=7.5Hz, 2H), 7.24(d, J=8.4Hz, 2H), 7.07(d, J=8.4Hz, 2H), 4.01(s, 2H). 13 C NMR (CDCl 3 , 300MHz, ppm) δ: 169.4, 156.6, 152.0, 133.6, 132.7, 132.0, 128.4, 126.8, 125.7, 122.3, 121.7, 118.3, 116.8, 40.0.
[0034] IR (KBr tablet) cm -1 : v 3058, 2839, 1625, 1591, 1498, 1438, 1266, 1216, 996, 760....
Embodiment 2
[0036]
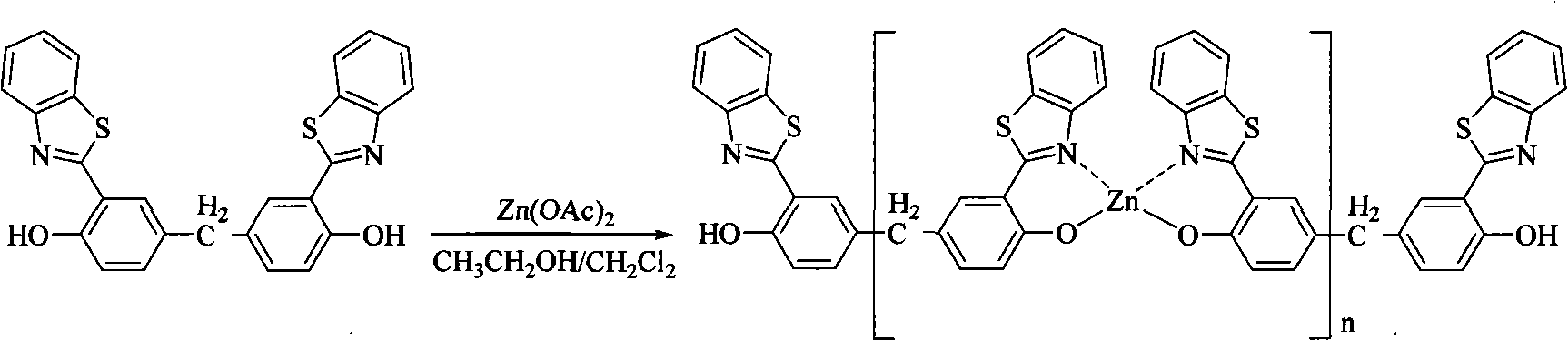
[0037] In the dichloromethane solution that dissolves the ligand of 0.05mmol (23.3mg), add dropwise the solution that has 0.055mmol (1.1eq, 7mg) Zn (OAc) 2 2H 2 2 mL of ethanol solution of O was added, and 0.1 mmol (2 eq, 14 μL) of triethylamine was added, and the reaction was stirred at room temperature for 24 h. Centrifuge after the reaction (5000rpm, 2min). The target compound was obtained by washing with ethanol and ether in sequence, and weighed after drying. The yield was 78%. IR (KBr tablet) cm -1 : v 3059, 1617, 1589, 1536, 1497, 1446, 1397, 1342, 1215, 998, 825, 756.
Embodiment 3
[0039]
[0040] Add 142mg (0.5mmol) of aldehyde, 131mg (1.05mmol, 2.1eq) of aminothiophenol, 2.5mL of DMSO into the sealed tube, and react at 185°C for 3h. After the reaction solution was cooled to room temperature, it was poured into 60mL water to form a white emulsion, and 5mL saturated saline was added to break the emulsion, CH 2 Cl 2 Extraction (15 mL x 3). The organic phases were combined, dried and concentrated, followed by column chromatography (petroleum ether / ethyl acetate=20:1) to obtain a light yellow solid, which was the ligand, with a yield of 46%. 1 H NMR (CDCl 3 , 300MHz, ppm) δ: 12.45(s, 2H), 8.01(d, J=8.1Hz, 2H), 7.89(d, J=8.1Hz, 2H), 7.64(d, J=8.1Hz, 2H), 7.52(t, J=7.4Hz, 2H), 7.41(t, J=7.7Hz, 2H), 7.30(dd, J=2.1, 10.8Hz, 2H), 7.07(d, J=8.7Hz, 2H), 1.81(s, 6H). 13 C NMR (CDCl 3, 300MHz, ppm) δ: 169.7, 156.2, 152.1, 141.6, 132.2, 126.8, 125.9, 125.6, 122.3, 122.2, 121.6, 117.9, 116.1, 41.9, 31.0.IR (KBr tablet) cm -1 : v 3448, 3052, 2967, 1625, 1594,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com