Pitavastatin calcium composition stabilized by using alkaline reagent and preparation method thereof
A technology of pitavastatin calcium and alkaline reagent, which is applied in the directions of drug combination, active ingredients of heterocyclic compounds, metabolic diseases, etc. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
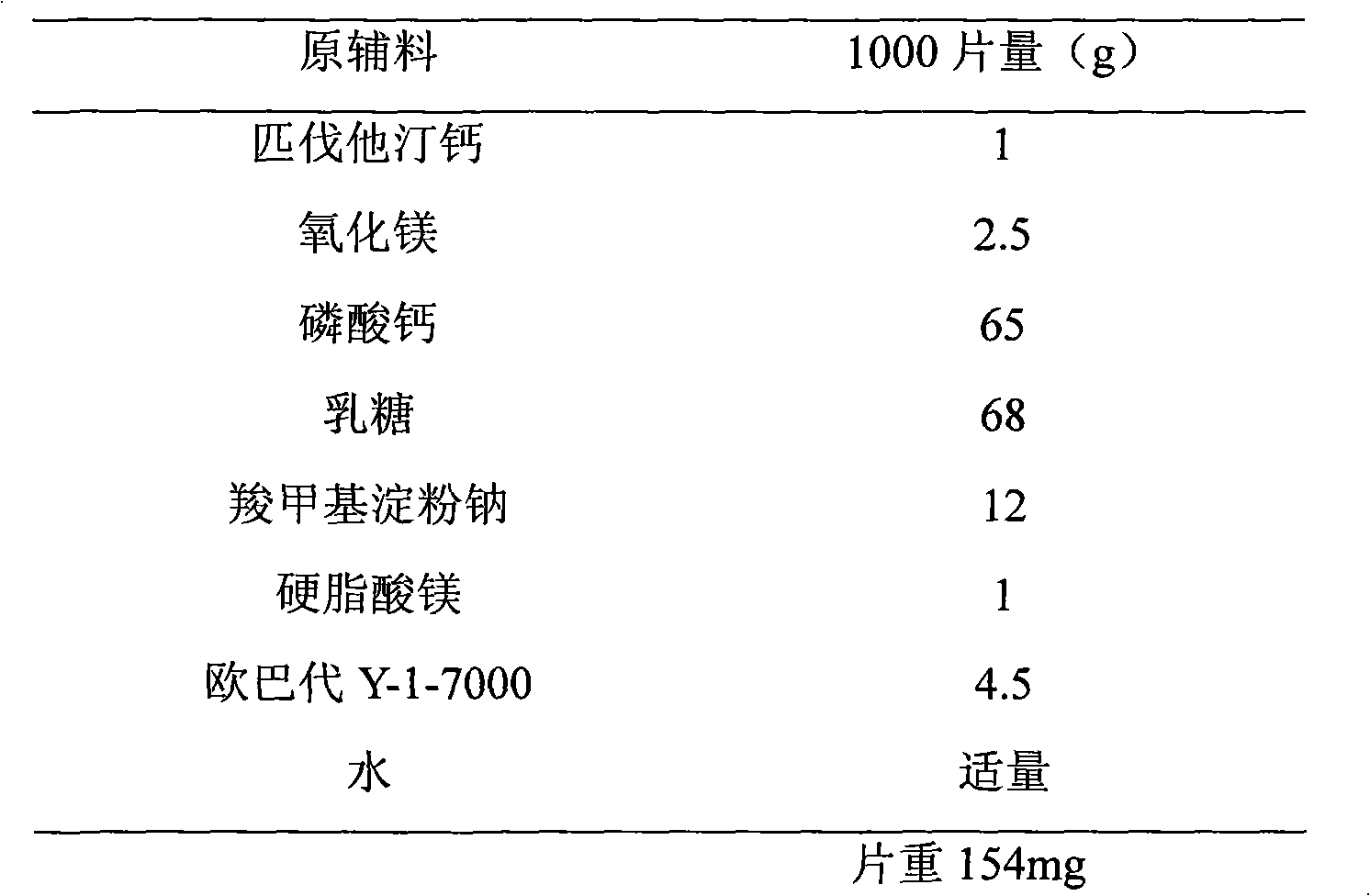
[0021] prescription:
[0022]
[0023] Preparation Process:
[0024] (1) Pitavastatin calcium was crushed through a 100-mesh sieve for use, and other excipients were passed through a 80-mesh sieve for use.
[0025] (2) The pitavastatin calcium and magnesium oxide of the recipe quantity are weighed and mixed with lactose in equal amounts, then the carboxymethyl starch sodium and calcium hydrogen phosphate of the prescription quantity are added, and the material I is mixed uniformly.
[0026] (3) Add an appropriate amount of water to material I, mix well, and pass through a 20-mesh sieve to obtain wet granules.
[0027] (4) Dry the wet granules in an oven at 50°C with air blast.
[0028] (5) The dry granules are granulated with a 20-mesh sieve, weighed, and the magnesium stearate in the prescription ratio is added and mixed evenly.
[0029] (6) Punch the sheet with a shallow concave of φ7mm.
[0030] (7) Prepare a coating solution with OPADRY Y-1-7000, coat and dry, and t...
Embodiment 2
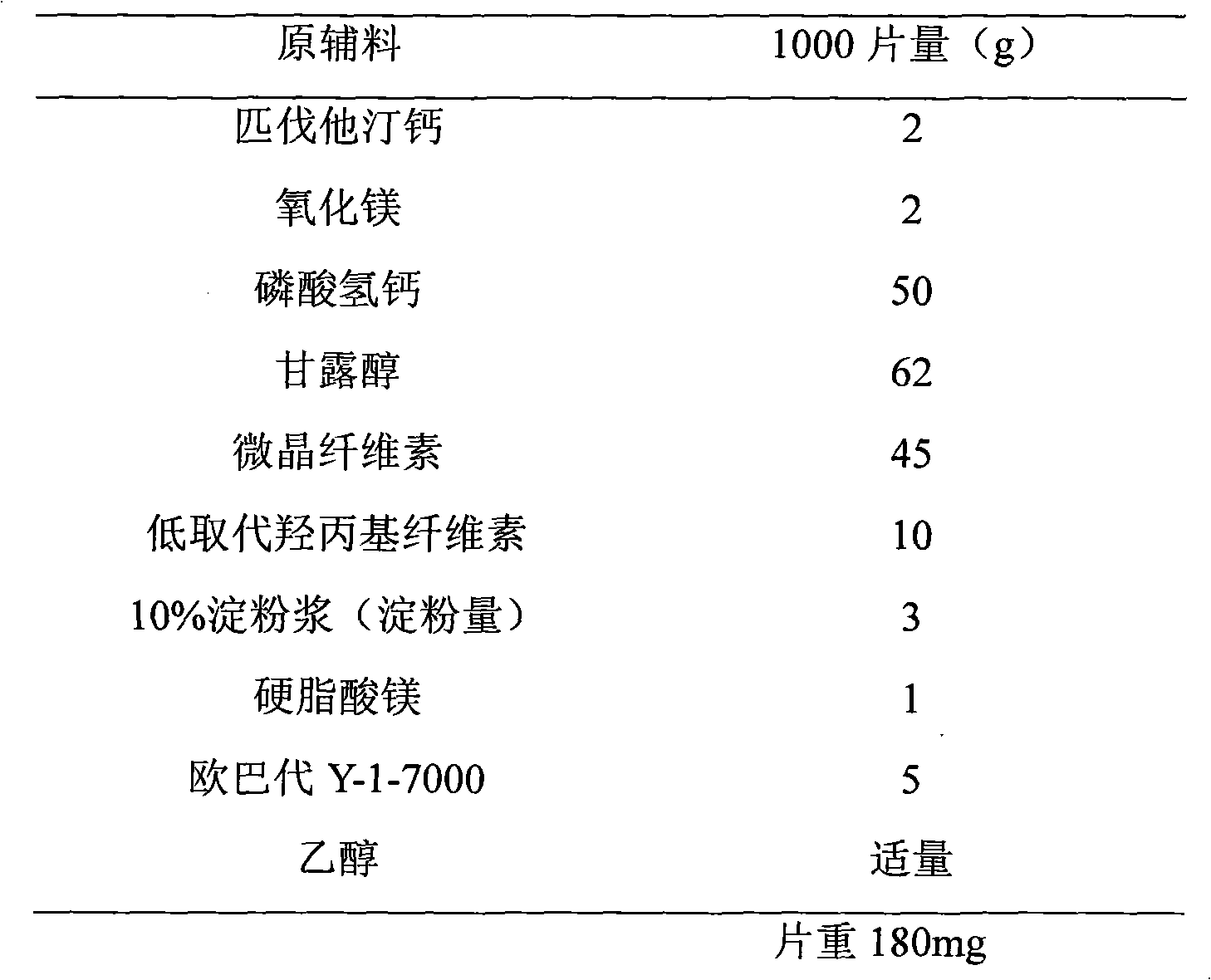
[0032] prescription:
[0033]
[0034] Preparation Process:
[0035] (1) Pitavastatin calcium was crushed through a 100-mesh sieve for use, and other excipients were passed through a 80-mesh sieve for use.
[0036] (2) Take by weighing the pitavastatin calcium of recipe quantity and magnesium oxide mix to obtain material I, mannitol, microcrystalline cellulose and low-substituted hydroxypropyl cellulose are mixed uniformly to obtain material II, and material I and material II are equally delivered Add and mix evenly to obtain material III.
[0037] (3) Add an appropriate amount of water to the material III, mix well, and pass through a 18-mesh sieve to obtain wet granules.
[0038] (4) Dry the wet granules in an oven at 50°C with air blast.
[0039] (5) The dry granules are granulated with a 20-mesh sieve, weighed, and the magnesium stearate in the prescription ratio is added and mixed evenly.
[0040] (6) Punch the sheet with a shallow concave of φ8mm.
[0041] (7) Pr...
Embodiment 3
[0043] prescription:
[0044]
[0045] Preparation Process:
[0046] (1) Pitavastatin calcium was crushed through a 100-mesh sieve for use, and other excipients were passed through a 80-mesh sieve for use.
[0047] (2) Take by weighing the pitavastatin calcium of recipe quantity and magnesium oxide mix to obtain material I, microcrystalline cellulose, sucrose, cross-linked povidone and micropowder silica gel mix homogeneously to obtain material II, and material I and material II are equally delivered Add and mix evenly to obtain material III.
[0048](3) Fill capsules with material III, and pack.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com