Method for separating and purifying amoxicillin trihydrate
A technology for amoxicillin and hydrate, which is applied in the field of separation and purification, can solve the problems of shortening the validity period and shelf life of amoxicillin preparations, yellowing, and affecting the storage time of amoxicillin trihydrate, and achieves easy industrial production and low cost , cheap effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 2L of methanol to 38L of amoxicillin hydrochloride aqueous solution (concentration: 0.6g / mL), add 11.4g of tetramethylammonium bromide, and use 25.87L of dilute ammonia water with a concentration of 10% in the range of 3°C-6°C Adjust the pH value of the solution to 4.8, keep the temperature of the solution at 1°C-4°C, grow the crystal for 1 hour, precipitate amoxicillin trihydrate, separate by centrifugation, wash the crystal with 2L of 3% methanol aqueous solution, and then wash with 3L of methanol , after centrifugal separation and vacuum drying, 23.32kg of amoxicillin trihydrate can be obtained, with a yield of 97.6%, and a content of 99.72% by HPLC external standard method.
[0033] HPLC method: use octadecyl bonded silica gel as filler; mobile phase A is 0.05mol / L phosphate buffer (get 0.05mol / L potassium dihydrogen phosphate solution, adjust pH value with 2mol / L sodium hydroxide solution to 5.0)-acetonitrile (99:1); mobile phase B is 0.05mol / L phosphate buffer...
Embodiment 2
[0037] Add 1.8L of ethanol to 23L of amoxicillin hydrochloride aqueous solution (concentration: 0.7g / mL), add 16.1g of diethylethanolammonium chloride, and dilute with a concentration of 10% in the range of 2°C to 6°C. Adjust the pH value of the solution to 4.82 with 17.57L of ammonia water, keep the temperature of the solution at 4°C-7°C to grow the crystal for 0.5h, precipitate amoxicillin trihydrate, centrifuge, wash the crystal with 1.8L of 5% ethanol aqueous solution, and then Then wash with 2.5 L of ethanol, centrifuge and vacuum dry to obtain 16.18 kg of amoxicillin trihydrate with a yield of 96% and a content of 99.81% by the HPLC external standard method. The HPLC method is the same as in Example 1.
Embodiment 3
[0039] Add 3L of isopropanol to 30L of amoxicillin hydrochloride aqueous solution (concentration: 0.5g / mL), add 1.5g of methyltriethylammonium chloride, and use concentration of 10% in the range of 3°C-6°C Adjust the pH value of the solution to 4.9 with 15.71L of dilute ammonia water, keep the temperature of the solution at 2°C-4°C and grow the crystal for 1.7h. Amoxicillin trihydrate is precipitated, separated by centrifugation, and washed with 2.6L of 10% isopropanol aqueous solution. The crystals were then washed with 2.8L of isopropanol, centrifuged and vacuum-dried to obtain 15.38 kg of amoxicillin trihydrate with a yield of 98% and a content of 99.85% by the HPLC external standard method. The HPLC method is the same as in Example 1.
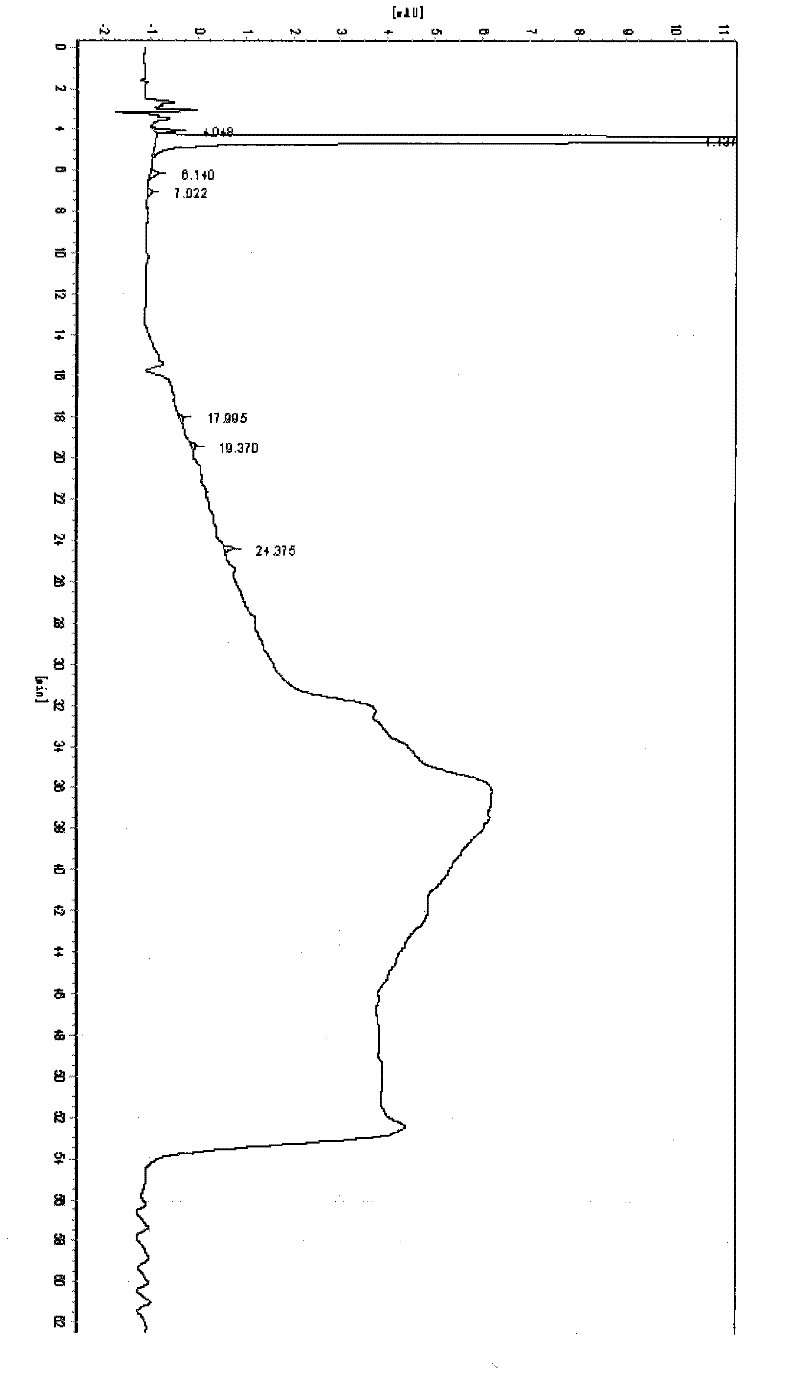
[0040] like figure 1 The Amoxicillin trihydrate HPLC collection of illustrative plates prepared for embodiment 3
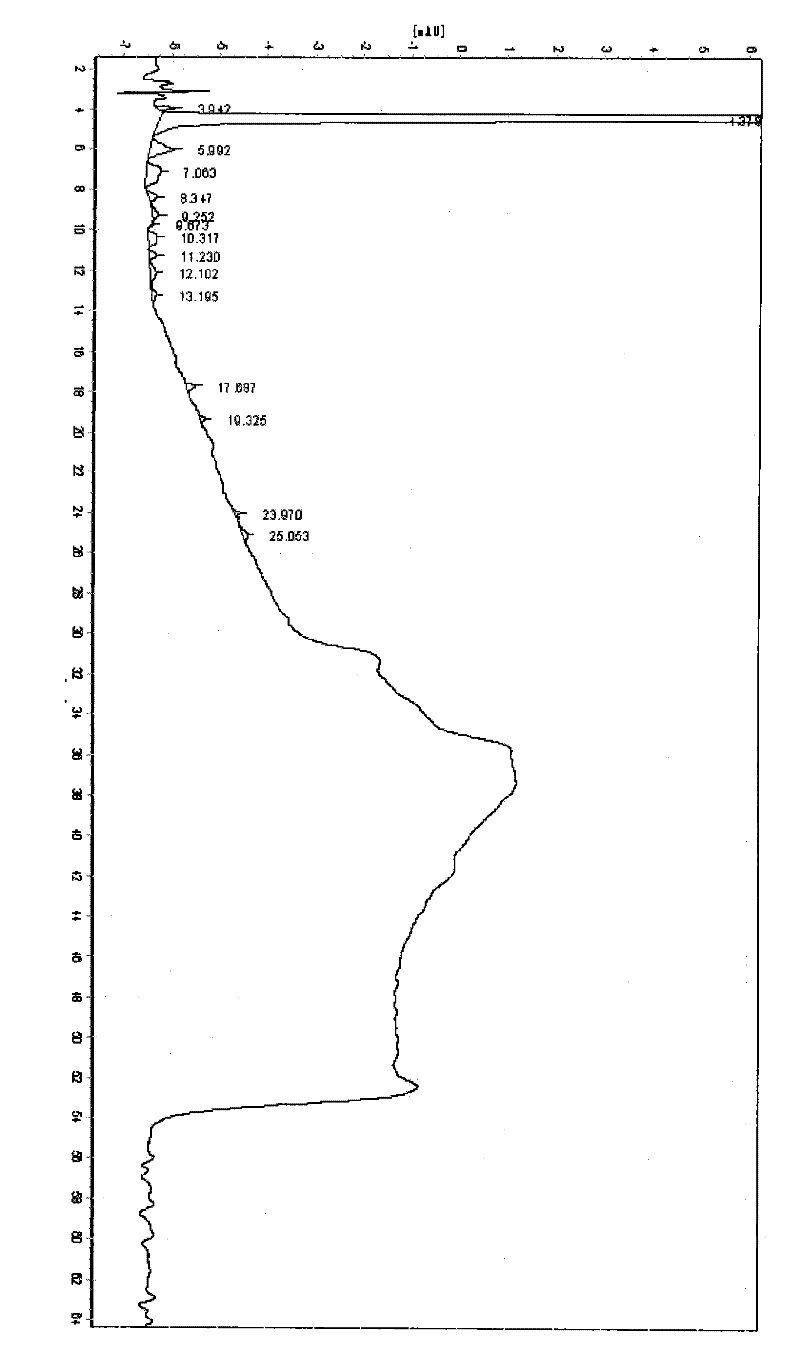
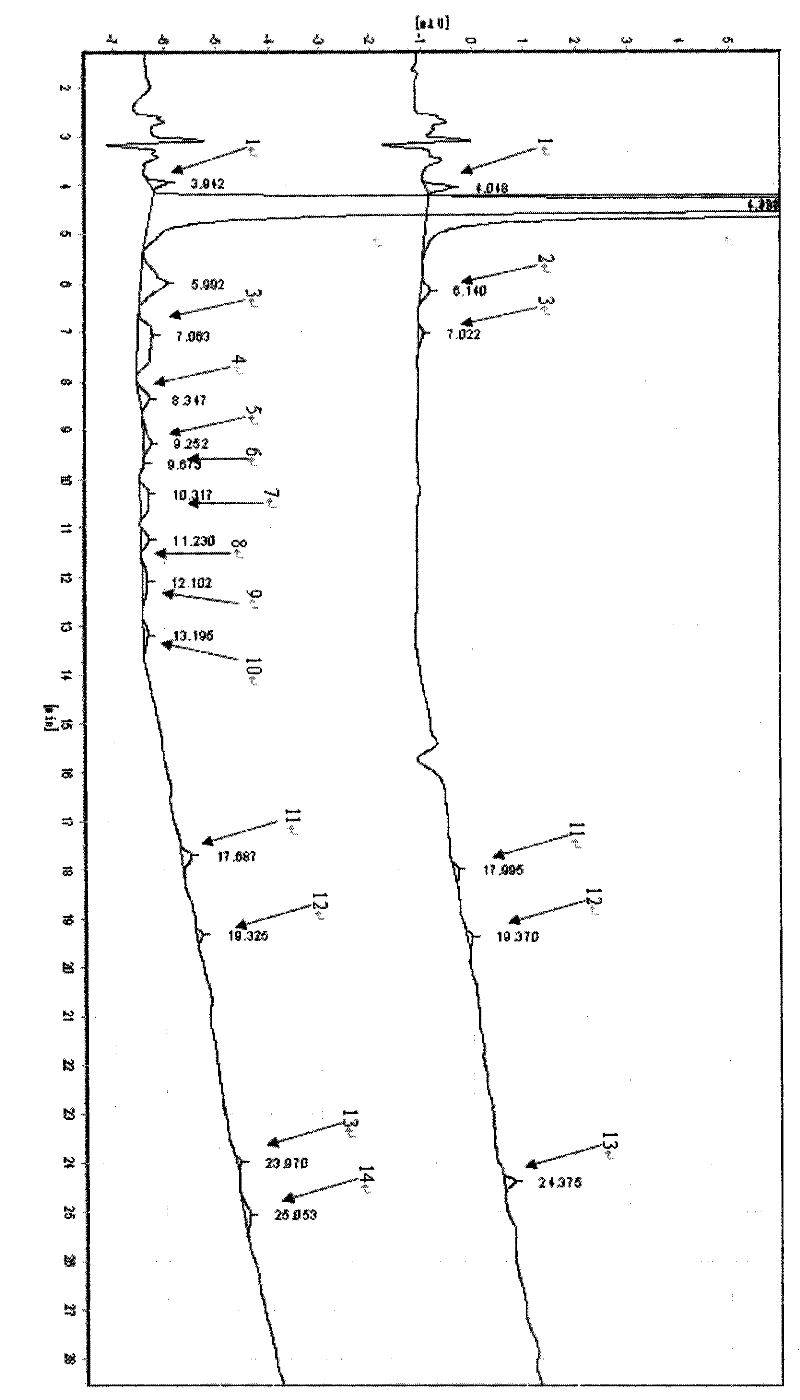
[0041] like image 3 It is the comparison chart of the HPLC spectrum of amoxicillin trihydrate prepared in Example 3 and C...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com