Recombinant BCG vaccine for tuberculosis prevention
A technology of recombinant BCG and BCG, applied in the direction of recombinant DNA technology, the use of vectors to introduce foreign genetic material, DNA / RNA fragments, etc., can solve problems such as safety concerns, and achieve excellent immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
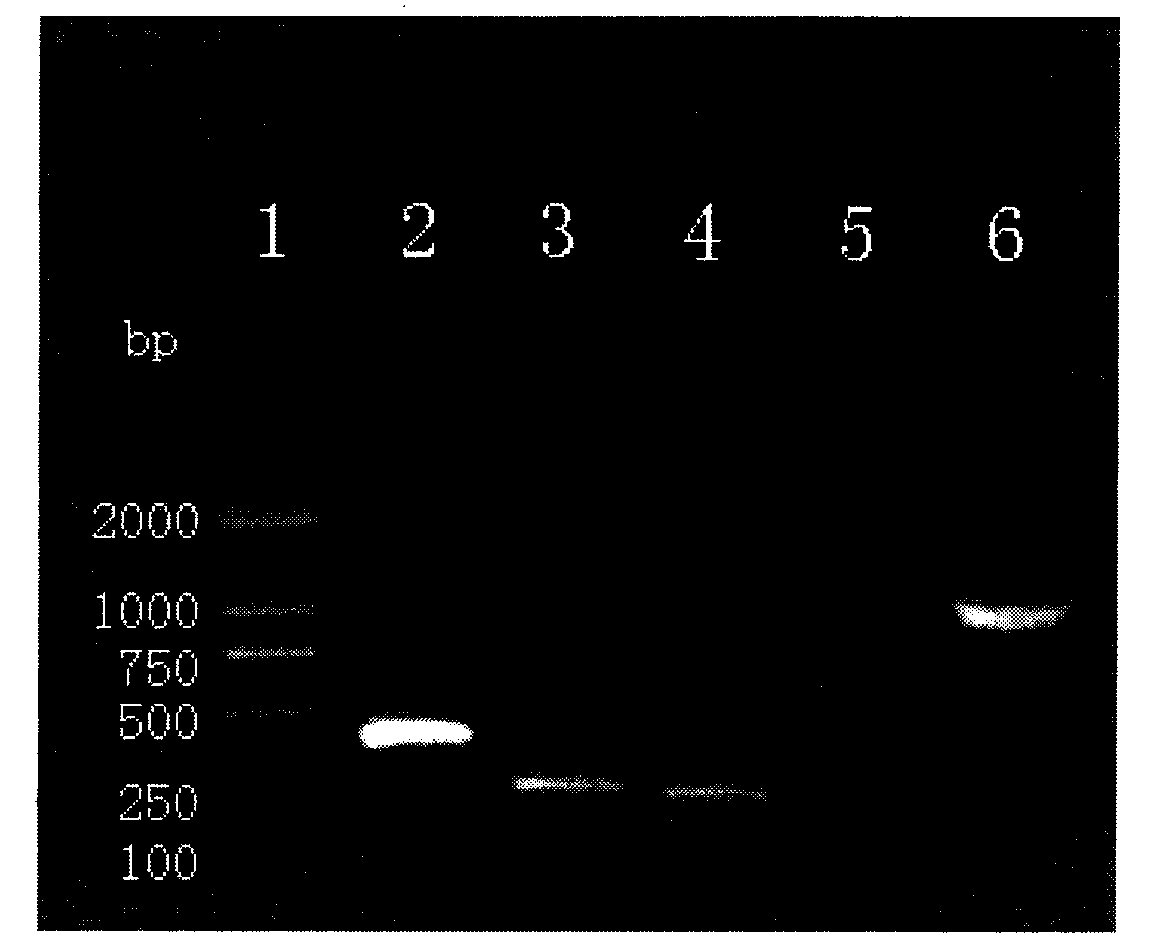
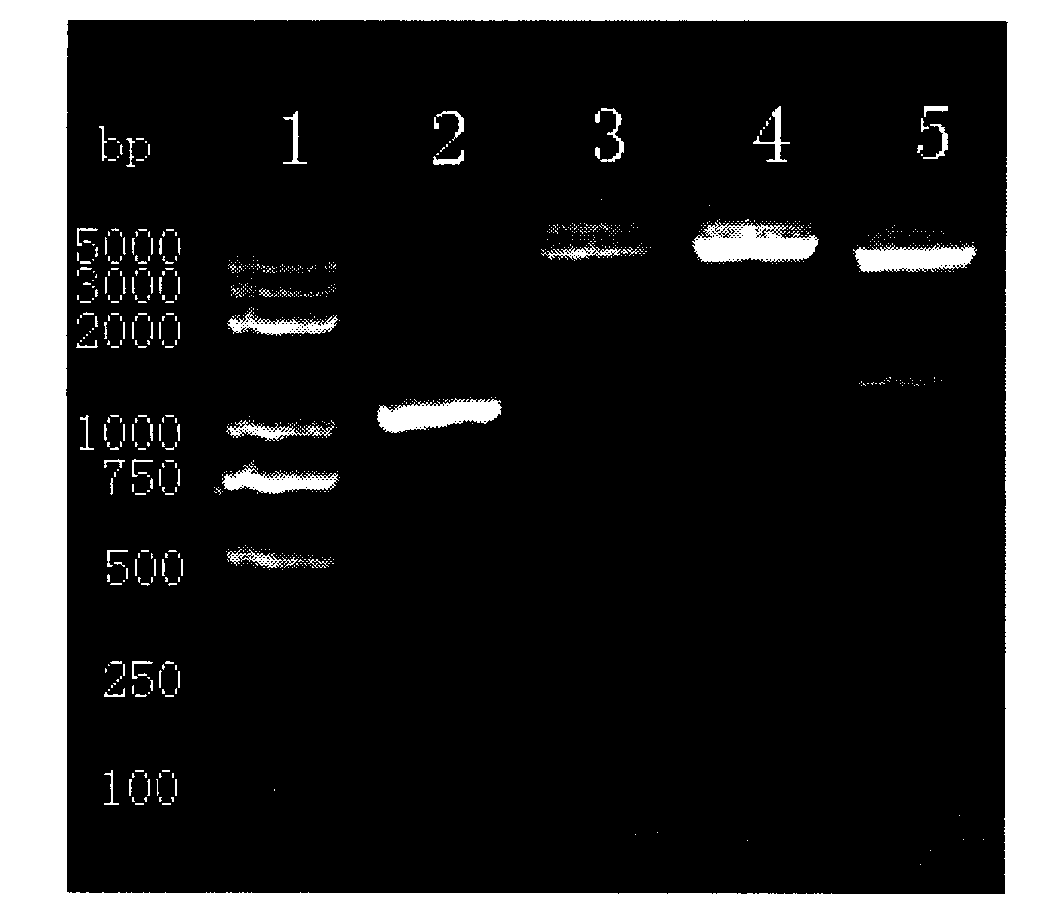
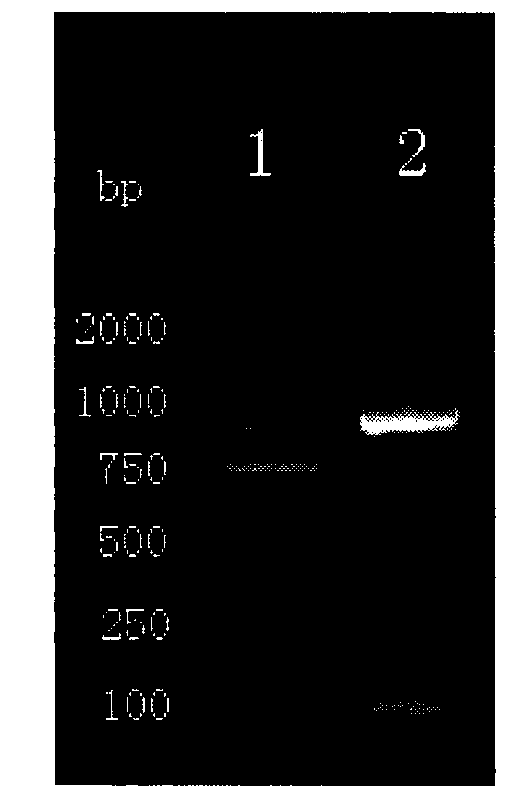
[0045] Example 1 Construction, expression and identification of recombinant BCG vaccine of GMCSF-CFP10-ESAT6 chimeric gene
[0046] 1 material
[0047] 1.1 Strains and plasmids
[0048] The BCG Shanghai strain was provided by Chengdu Institute of Biological Products, the plasmid pMV361 was kept in our laboratory, and the pORF-hGMCSF plasmid was purchased from Invitrogen.
[0049] 1.2 Main reagents
[0050] prime STAR TM HS DNA polymerase, dNTP and DNA ligation kits are products of Takara; EcoRI, HindIII and protein molecular weight standards were purchased from Jingmei; Omega plasmid extraction kit, PCR product purification kit and gel recovery kit, bacterial genome extraction reagent The box was purchased from Baoxin Bio; the human GM-CSF monoclonal antibody was a product of Abcam; the HRP enzyme-labeled goat anti-mouse IgG and ECL color reagent kit was purchased from Beijing Zhongshan Company.
[0051] 1.3 Main instruments
[0052] The PCR machine and the electric transfer machine we...
Embodiment 2
[0082] Example 2 Recombinant BCG vaccine rBCG: GMCSF-CFP10-ESAT6 immunogenicity study
[0083] 1 material
[0084] 1.1 Experimental animals BALB / C mice were purchased from the Experimental Animal Center of West China Campus of Sichuan University.
[0085] 1.2 The main reagents IFN-γ, IL-4 ELISA detection kits were purchased from R&D company, PE-labeled CD 4 And FITC labeled CD 8 Anti-mouse primary antibodies were purchased from eBioscience, and RPMI1640 medium and fetal bovine serum were purchased from Gibco.
[0086] 1.3 Main instruments: FACSCalibur flow cytometer (BD Biosciences), microplate reader (Bio-Rad).
[0087] 2 method
[0088] 2.1 Animal immunity
[0089] Take 32 BALB / C mice aged 6 weeks (male or female), 8 mice in each group, randomly divided into PBST group, BCG group, rBCG:361 group and rBCG:GMCSF-CFP10-ESAT6 group, each group is adaptable After feeding for 1 week, PBST (0.05% Tween80), BCG and rBCG (5×10 6 CFU per mouse, diluted with PBST to 0.1 mL), immunized once. Afte...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com