Microsphere injection of medicinal composition of cefotiam hydrochloride and anhydrous sodium carbonate
A technology of cefotiam hydrochloride and anhydrous sodium carbonate, which is applied in the field of medicine and can solve the problems of increasing pharmacological effects and limiting the number of people taking medication
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
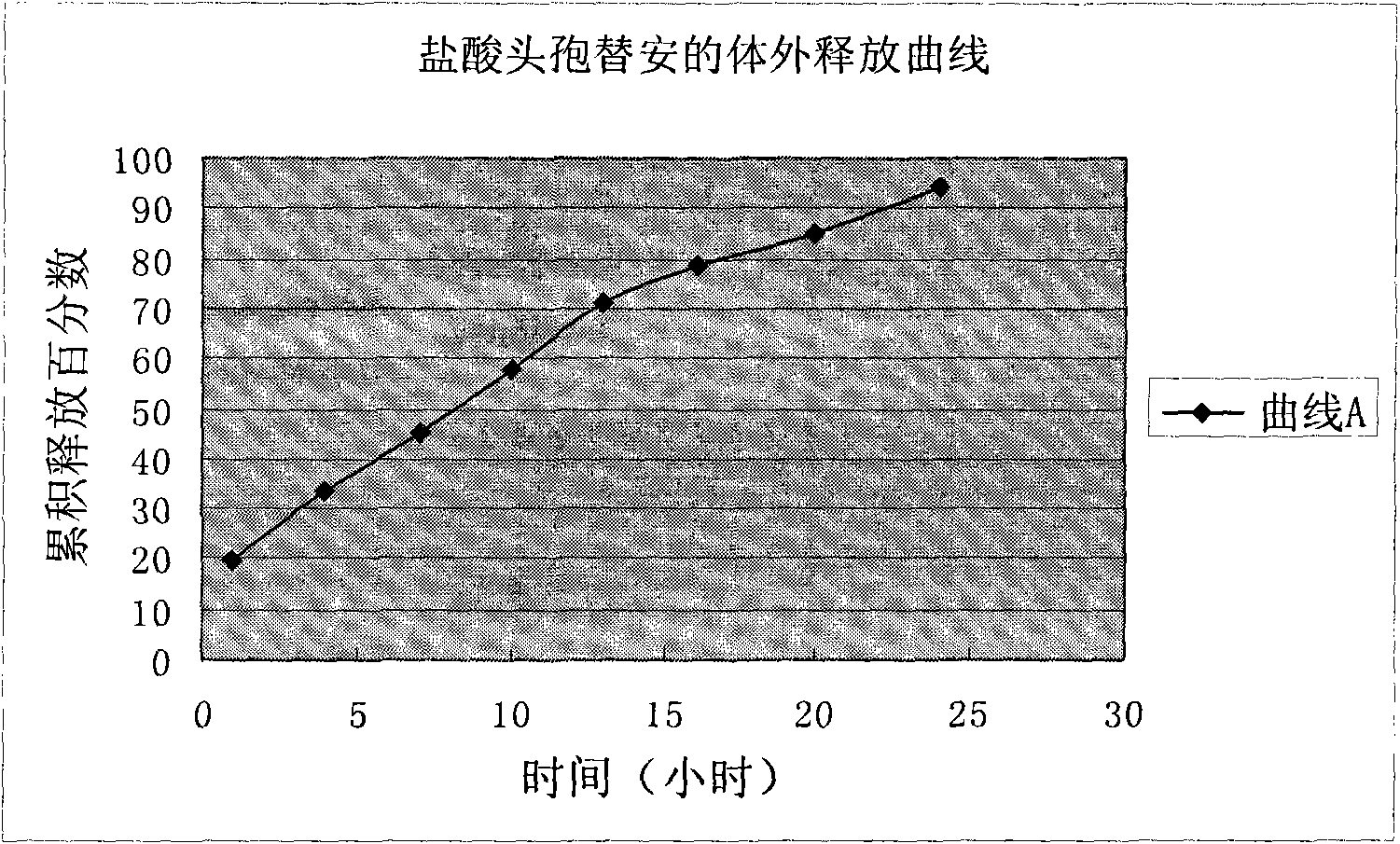
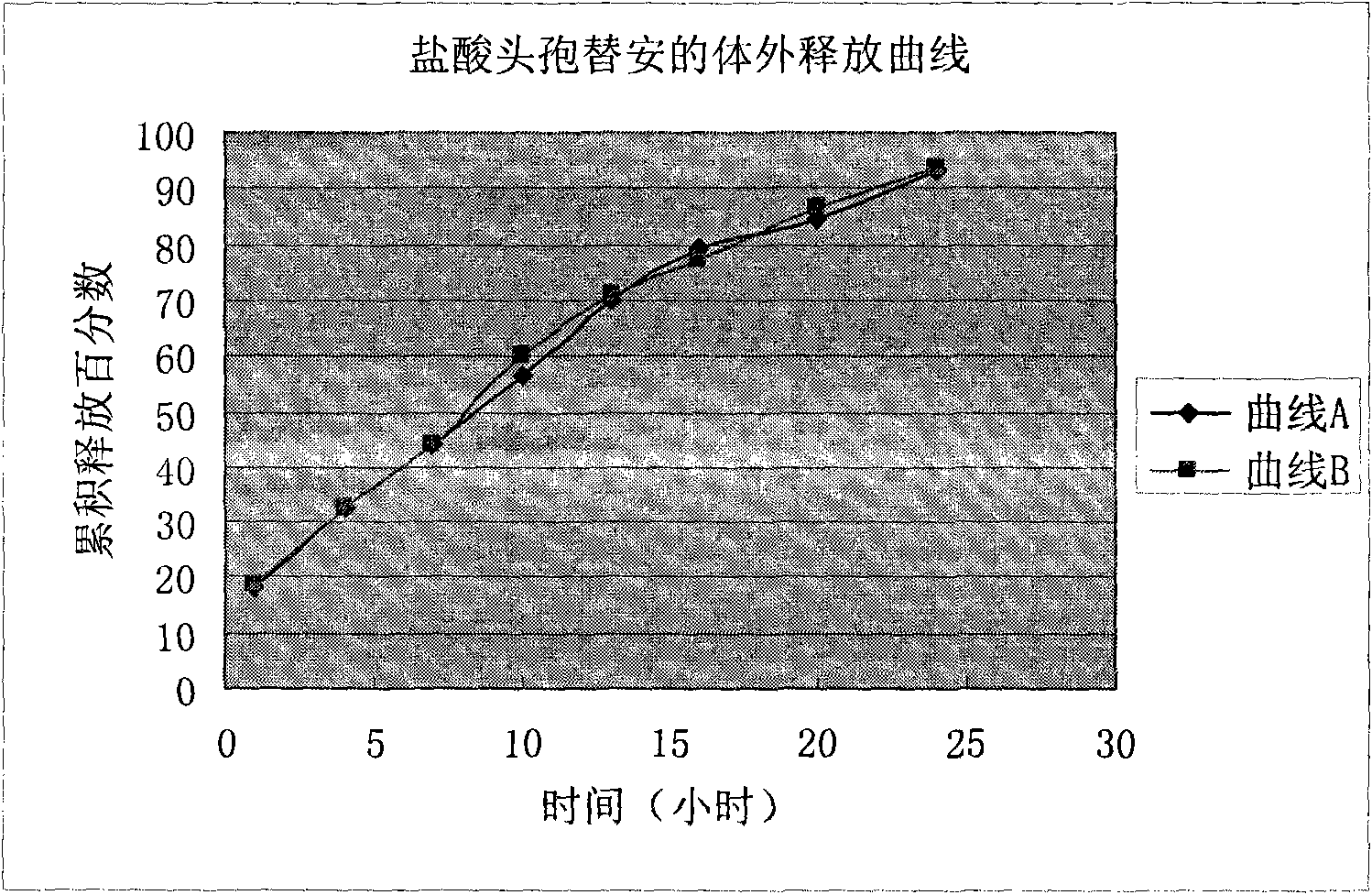
Image
Examples
Embodiment 1
[0060] The preparation of embodiment 1 cefotiam hydrochloride / anhydrous sodium carbonate injection
[0061] Prescription: (100 bottles)
[0062] Cefotiam Hydrochloride 50g
[0063] Anhydrous sodium carbonate 7.5g
[0064] PLGA 125g
[0065] Glycerin 40g
[0066] Fatty Acid Sorbitan 80 50g
[0067] Sorbitol 250g
[0068] making process:
[0069] (1) 50g cefotiam hydrochloride, 40g glycerol and 250g sorbitol are dissolved in 1000ml water for injection to obtain the aqueous phase;
[0070] (2) 125g PLGA and 50g fatty acid sorbitan 80 are dissolved in 500ml of dichloromethane and toluene mixed solvent with a volume ratio of 2:1 to obtain an oily phase;
[0071] (3) Slowly drop the water phase obtained above into the oil phase under stirring conditions, control the dropping speed to 5ml / min, stir for 10min after dropping, then transfer to a high-speed homogenizer and stir at a high speed for 3 times at a speed of 15000r / min, each 10min, to obtain a uniform white emulsion; ...
Embodiment 2
[0074] The preparation of embodiment 2 cefotiam hydrochloride / anhydrous sodium carbonate injection
[0075] Prescription: (100 bottles)
[0076] Cefotiam Hydrochloride 100g
[0077] Anhydrous sodium carbonate 20g
[0078] PLGA 500g
[0079] Glycerin 150g
[0080] Fatty Acid Sorbitan 80 300g
[0081] Sorbitol 1000g
[0082] making process:
[0083] (1) 100g cefotiam hydrochloride, 150g glycerol and 1000g sorbitol are dissolved in 3000ml water for injection to obtain the aqueous phase;
[0084] (2) 500g PLGA and 300g fatty acid sorbitan 80 are dissolved in 2000ml volume ratio and be in the methylene chloride and toluene mixed solvent of 2: 1, obtain oily phase;
[0085] (3) Slowly drop the water phase obtained above into the oil phase under stirring conditions, control the dropping speed to be 10ml / min, stir for 30min after dropping, then transfer to a high-speed homogenizer for high-speed stirring for 5 times at a speed of 15000r / min, each 5min, to obtain a uniform whi...
Embodiment 3
[0088] The preparation of embodiment 3 cefotiam hydrochloride / anhydrous sodium carbonate injection
[0089] Prescription: (100 bottles)
[0090] Cefotiam Hydrochloride 200g
[0091] Anhydrous sodium carbonate 40g
[0092] PLGA 500g
[0093] Glycerin 300g
[0094] Fatty Acid Sorbitan 80 200g
[0095] Sorbitol 2000g
[0096] making process:
[0097] (1) 200g cefotiam hydrochloride, 300g glycerol and 2000g sorbitol are dissolved in 5000ml water for injection to obtain the aqueous phase;
[0098](2) 500g PLGA and 200g fatty acid sorbitan 80 are dissolved in 2000ml of dichloromethane and toluene mixed solvent with a volume ratio of 2:1 to obtain an oily phase;
[0099] (3) Slowly drop the water phase obtained above into the oil phase under stirring conditions, control the dropping speed to 8ml / min, stir for 20min after dropping, then transfer to a high-speed homogenizer for high-speed stirring 4 times at a speed of 15000r / min, 7min each time, to obtain a uniform white emul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| encapsulation rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com