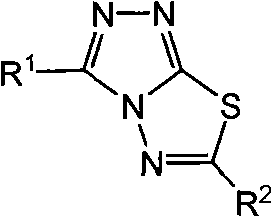
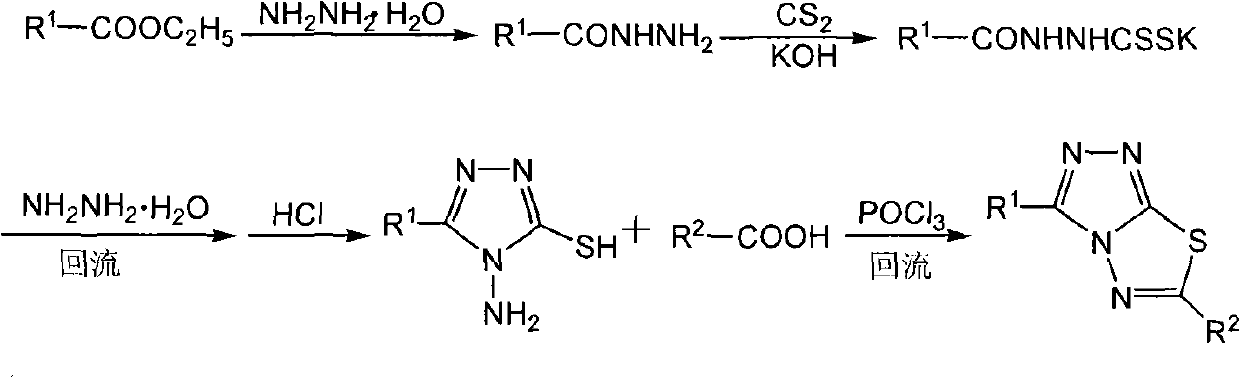
Triazolothiadiazole compound and its preparation method and use
A technology of thiadiazoles and thiadiazole derivatives, which is applied in the field of heterocyclic compounds and can solve the problems of insufficient research reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Preparation and structure identification of compound 3-(4-methyl-1,2,3-thiadiazol-5-yl)-4-amino-5-mercapto-1,2,4-s-triazole
[0044] Add 6.25 g (0.1 mole) of 80% hydrazine hydrate to 50 ml of absolute ethanol, and slowly add 17.2 g (0.1 mole) of 4-methyl-1,2,3-thiadiazole-5-carboxylic acid dropwise with stirring Ethyl ester, after dripping, stir vigorously, about 15 minutes, a large amount of solids precipitated instantly, filtered with suction and washed with absolute ethanol, spin-dried the filtrate to obtain a yellow solid, washed with petroleum ether, combined the solids, and dried under infrared light to obtain an orange-yellow 4- Methyl-1,2,3-thiadiazole-5-carboxhydrazide was 14.9 g, and the yield was 94.2%; 11.6 g (0.07 mol) of 4-methyl-1,2,3-thiadiazole-5 -Formyl hydrazide was added to 7.5 g (0.11 mol) of 82% KOH in 150 ml of absolute ethanol solution, stirred to dissolve, and then CS was added dropwise 2 8.36 g (0.11 mol), an orange-yellow solid gradually precipit...
Embodiment 2
[0046] Compound YZK-a: 3-phenyl-6-(4-methyl-1,2,3-thiadiazol-5-yl)s-triazolo-[3,4-b]-1,3,4 -Synthesis and structure identification of thiadiazole
[0047] 1.5 mmoles of 3-phenyl-4-amino-5-mercapto-1,2,4-s-triazole and 1.5 mmoles of 4-methyl-1,2,3-thiadiazole-5-carboxylic acid were added to Add 10 ml POCl to a 25 ml round bottom flask 3 , Magnet stirring, oil bath heating to maintain the temperature 110-120 degrees, reflux for 5 hours, the reaction is complete, under ice water cooling, slowly add ice water dropwise, a large amount of solid precipitation, let stand overnight, suction filtration, saturated NaHCO 3 The product was washed with solution, then washed with water to neutrality, and dried to obtain a crude product with a yield of 87%. Use DMF-C 2 H 5 OH is recrystallized to obtain green needle-like crystals; melting point: 173-175 degrees, 1 HNMR(δ, DMSO-d 6 ): 7.563-8.246 (m, 5H, ArH), 3.003 (s, 3H, thiadiazolyl-CH 3 ).
Embodiment 3
[0049] Compound YZK-b: 3-phenyl-6-(5-methyl-1,2,3-thiadiazol-5-yl)s-triazolo-[3,4-b]-1,3,4 -Synthesis and structure identification of thiadiazole
[0050] 1.0 mmol of 3-phenyl-4-amino-5-mercapto-1,2,4-s-triazole and 1.0 mmol of 5-methyl-1,2,3-thiadiazole-4-carboxylic acid were added to Add 10 ml of POCl3 to a 25 ml round bottom flask, stir with a magnet, heat the oil bath to maintain the temperature at 110-120 degrees, reflux for 5 hours, after the reaction is complete, under ice water cooling, slowly add ice water dropwise, a large amount of solids precipitate out, Set overnight, filter with suction, use saturated NaHCO 3 The product is washed with solution, then washed with water to neutrality, and dried to obtain a crude product with a yield of 60%. Use DMF-C 2 H 5 OH is recrystallized to obtain off-white powdery crystals; melting point: 236-239 degrees, 1 H NMR(δ,DMSO-d 6 ): 7.573-8.297 (m, 5H, ArH), 3.062 (s, 3H, thiadiazolyl-CH 3 ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com