Matrix of suppository or paste medicine for treating prostatitis and preparation method thereof
A technology of matrix and ointment, applied in the directions of suppository delivery, drug combination, pharmaceutical formulation, etc., can solve the problems of destroying the uniformity and appearance of preparations, separation of matrix phases, etc., and achieve the effect of good production smoothness and quality characteristics.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
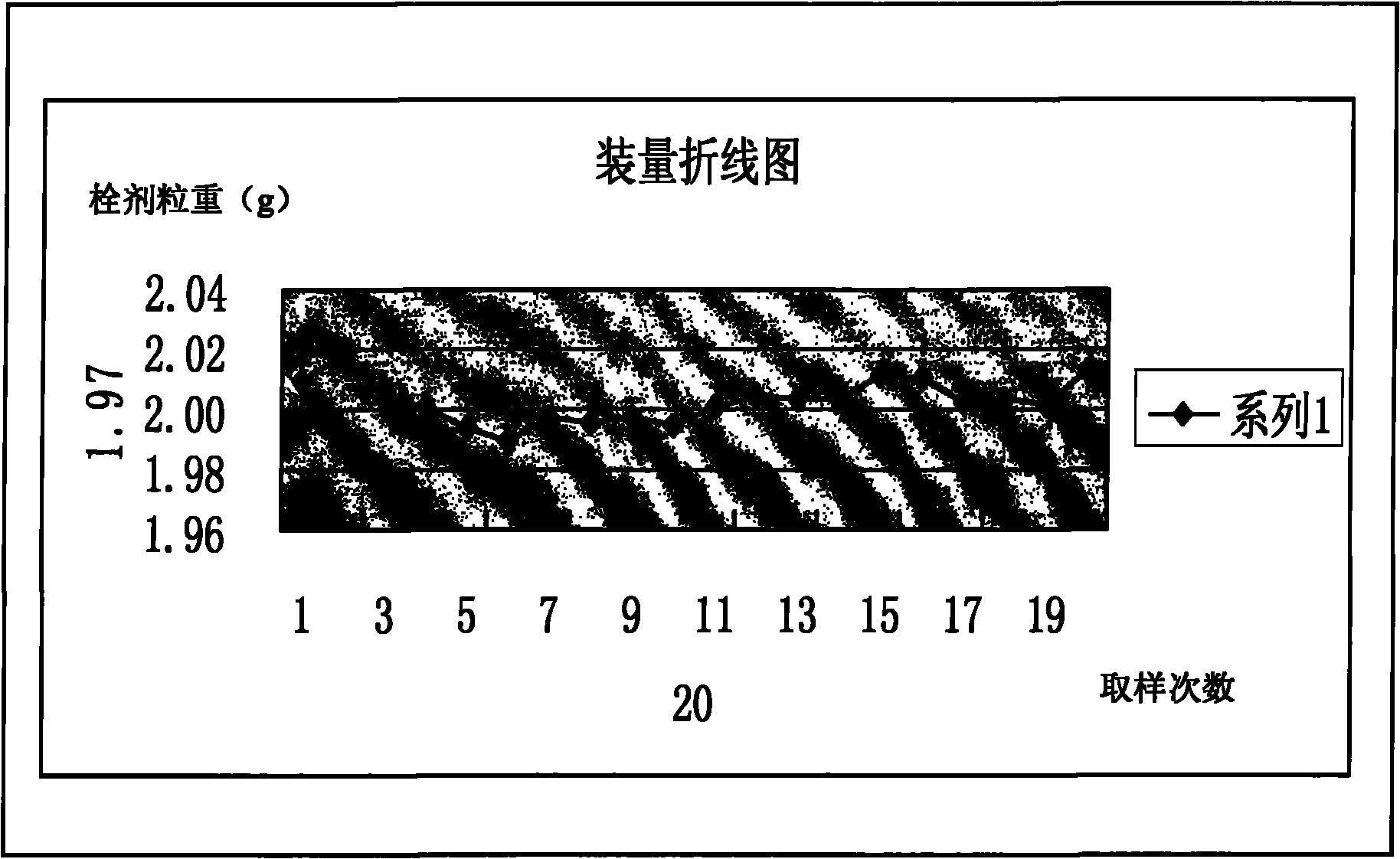
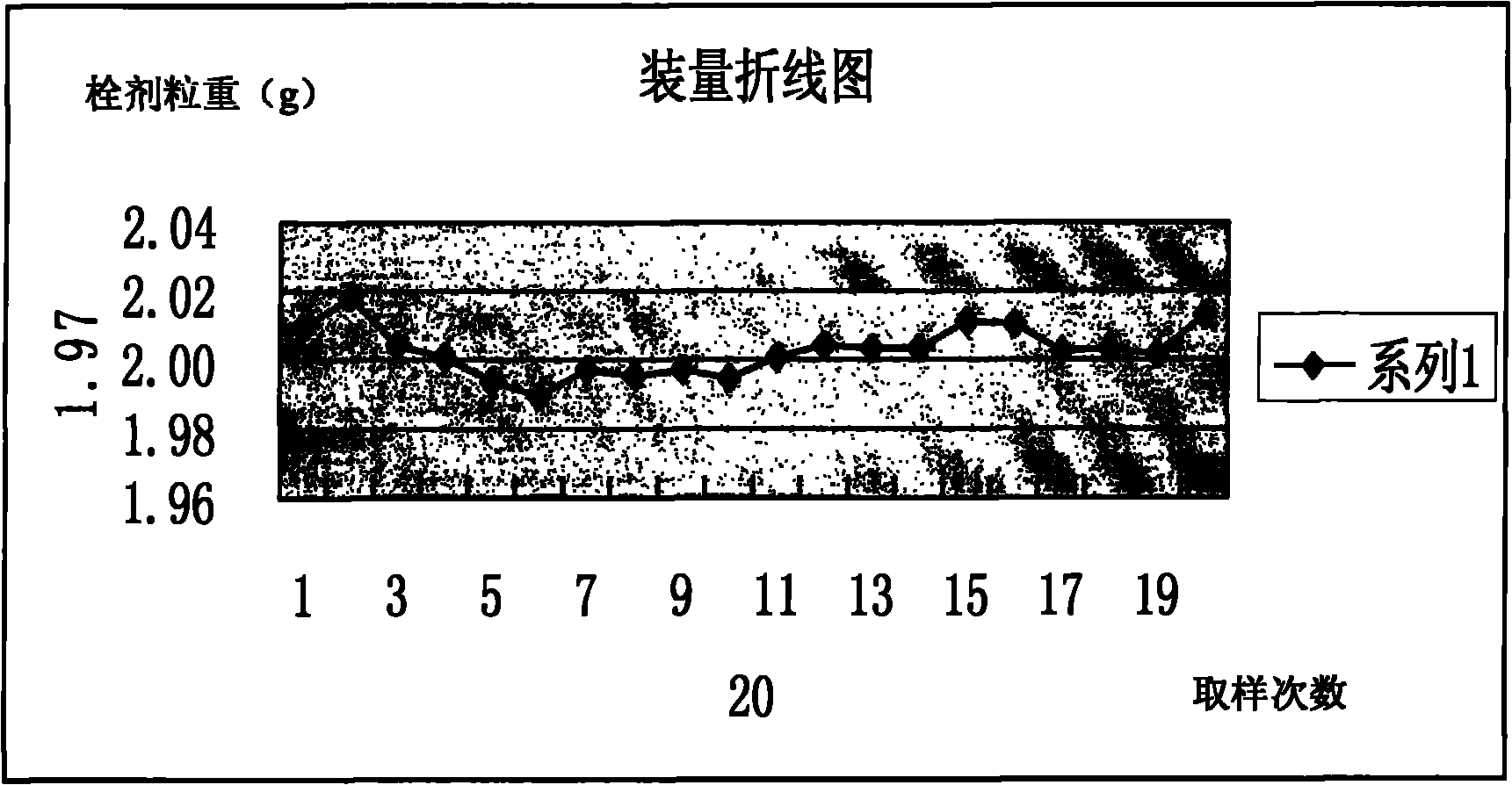
Image
Examples
Embodiment 1
[0038]Embodiment 1 (composition A)
[0039] According to the clinically commonly used suppository specification of a certain medicine is 2g / grain, 0.6kg of medicinal powder needs to be added to prepare 1000 pills, and the medicinal powder needs to be proportioned with 10% of its weight in purified water. According to the test, to meet the requirements of preparation melting time limit, hardness and delamination resistance, the matrix composition is formulated as follows: the total weight of the matrix is the weight of 1000 suppositories-medicine powder weight-pure water weight, that is: (1000 * 2-660) ( unit is g)
[0040] Various fatty acid glycerides 1183 88.3%
[0041] Macrogol 400 Monolaurate 67 5%
[0042] Macrogol 400 Dilaurate 53.6 4%
[0043] Glyceryl behenate 33.5 2.5%
[0044] Appropriate amount of preservatives
[0045]
[0046] The total weight of the matrix is 1340 (in g) 100%
[0047] A variety of ...
Embodiment 2
[0048] Example 2 (composition B)
[0049] According to the commonly used clinical suppository specification of a certain medicine is 2g / granule, 0.7kg of medicinal powder needs to be added to prepare 1000 capsules, and the medicinal powder needs to be mixed with 15% of its weight in purified water. According to the test, it meets the requirements of melting time limit, hardness and anti-stratification of the preparation , formulate matrix composition as follows: the total weight of matrix is the weight of 1000 suppositories-medicine powder weight-pure water weight, namely: (1000 * 2-700-105) (unit is g)
[0050] Various fatty acid glycerides 1055 88.3%
[0051] Macrogol 400 Monopalmitate 47.8 4%
[0052] Macrogol 400 dipalmitate 71.7 6%
[0053] Glyceryl Behenate 17.9 1.5%
[0054] Appropriate amount of preservatives
[0055]
[0056] The total weight of the matrix is 1195 (in g) 100%
[0057] A variety of fatty...
Embodiment 3
[0058] Embodiment 3 (composition C)
[0059] According to the commonly used clinical suppository specification of a certain drug is 2g / grain, 0.5kg of medicinal powder needs to be added to prepare 1000 pills, and the medicinal powder needs to be mixed with 8% of its weight in purified water. According to the test, it meets the requirements of the preparation melting time limit, hardness, and anti-stratification , formulate matrix composition as follows: the total consumption of matrix is the weight of 1000 suppositories-medicine powder weight-pure water weight, namely: 1000 * 2-500-40 (unit is g)
[0060] Various fatty acid glycerides 1311 89.8%
[0061] Macrogol 400 Monostearate 73 5%
[0062] Macrogol 400 Distearate 51.1 3.5%
[0063] Glyceryl behenate 21.9 1.5%
[0064] Appropriate amount of preservatives
[0065]
[0066] The total weight of the matrix is 1460 (in g) 100%
[0067] A variety of fatty acid glyc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| viscosity | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com