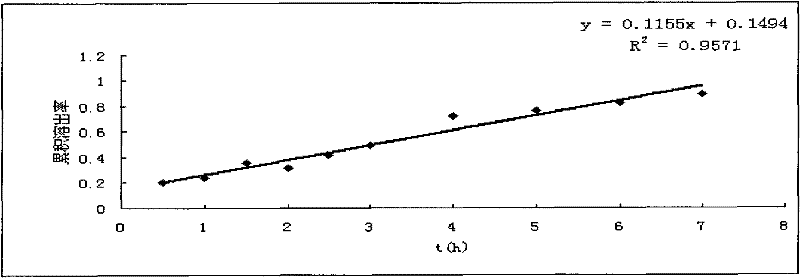
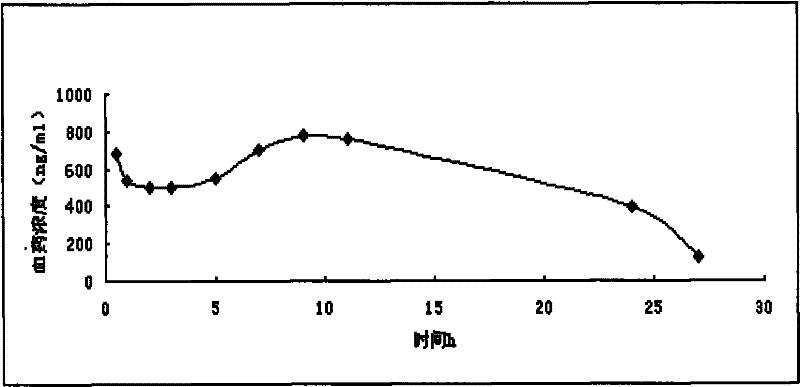
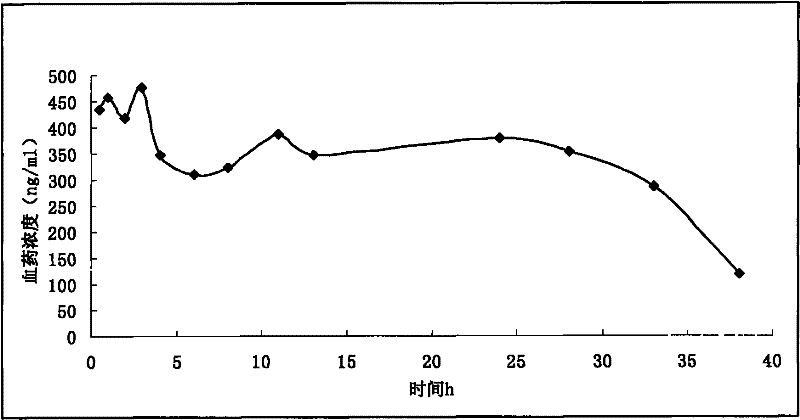
Morphine sulfate sustained/controlled-release suppository and preparation method thereof
A morphine sulfate and suppository technology, which is applied in the directions of suppository delivery, pharmaceutical formulations, and non-active ingredients medical preparations, etc., can solve the problems of inability to achieve sustained and controlled release, unstable blood drug concentration, and short effect time, and achieve reliable curative effect. , the effect of reducing the frequency of administration and reducing the dosage of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The morphine sulfate sustained-release suppository provided in this embodiment contains the following percentages by weight:
[0047] Morphine sulfate 3.48%;
[0048] Poloxamer 188 24.00%;
[0049] Poloxamer 407 26.40%;
[0050] BHT 0.02%;
[0051] Glycerin 40.50%
[0052] Azone 4.80%;
[0053] Carbomer 0.80%.
[0054] The preparation method of above-mentioned morphine sulfate suppository, comprises the steps:
[0055] A. Pass the main drug morphine sulfate through a 180 or more mesh sieve; the auxiliary materials include F68, F127, carbomer, 2,6-di-tert-butyl p-cresol (BHT), and the auxiliary materials pass through a 100 mesh or more mesh sieve respectively ,spare;
[0056] B. F68, F127, BHT, morphine sulfate, carbomer, stir and mix well, heat to 60-80°C to melt, stir and mix well;
[0057] C. Stir the molten substrate in step B at 60-75°C, add glycerin and azone and keep warm and stir evenly;
[0058] D. Stir the mixture in step C at a constant temperature of 6...
Embodiment 2
[0075] The morphine sulfate sustained-release suppository provided in this embodiment contains the following percentages by weight:
[0076] Morphine sulfate 5.94%;
[0077] Poloxamer 188 28.05%;
[0078] Poloxamer 407 25.25%;
[0079] BHT 0.02%;
[0080] Glycerin 35.84%
[0081] Azone 4.20%;
[0082] Carbomer 0.70%.
[0083] The preparation method of above-mentioned morphine sulfate suppository, comprises the steps:
[0084] A. Pass the main drug morphine sulfate through a 180 or more mesh sieve; the auxiliary materials include F68, F127, carbomer, 2,6-di-tert-butyl p-cresol (BHT), and the auxiliary materials pass through a 100 mesh or more mesh sieve respectively ,spare;
[0085] B. F68, F127, BHT, morphine sulfate, and carbomer are stirred and mixed, then heated to 70°C to melt, and stirred again;
[0086] C. Add glycerin and azone to the melted substrate in step B under stirring at 40-45°C and keep warm and stir evenly;
[0087] D. Stir the mixture in step C to a ...
Embodiment 3
[0106] The morphine sulfate sustained-release suppository provided in this embodiment contains the following percentages by weight:
[0107] Morphine sulfate 7.70%;
[0108] Poloxamer 188 21.76%;
[0109] Poloxamer 407 28.29%;
[0110] BHT 0.02%;
[0111] Glycerin 37.16%
[0112] Azone 4.35%;
[0113] Carbomer 0.72%
[0114] The preparation method of above-mentioned morphine sulfate suppository, comprises the steps:
[0115] A. Pass the main drug morphine sulfate through a 180 or more mesh sieve; the auxiliary materials include F68, F127, carbomer, 2,6-di-tert-butyl p-cresol (BHT), and the auxiliary materials pass through a 100 mesh or more mesh sieve respectively ,spare;
[0116] B. F68, F127, BHT, morphine sulfate, and carbomer are stirred and mixed, then heated to 70°C to melt, and stirred again;
[0117] C. Add glycerin and azone to the melted substrate in step B under stirring at 40-45°C and keep warm and stir evenly;
[0118] D. Stir the mixture in step C at a c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com