Method for separating, enriching and identifying macromolecular weight protein
A high molecular weight, separation and enrichment technology, applied in the field of biochemical analysis, can solve the problems of difficult separation and analysis of large molecular weight proteins, and achieve the effects of good mass spectrometry compatibility, good reproducibility and large pore size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Example 1 Research on the Optimum Separation Molecular Weight Range of Protein High Molecular Weight End in Low Melting Point Agarose / Polyacrylamide Mixed Gel
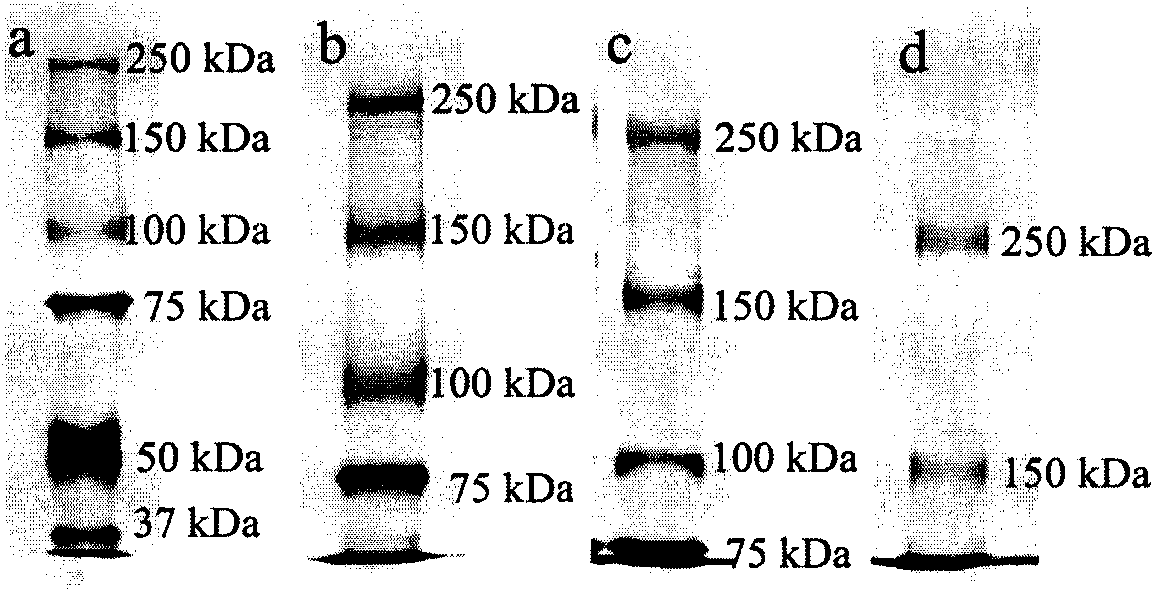
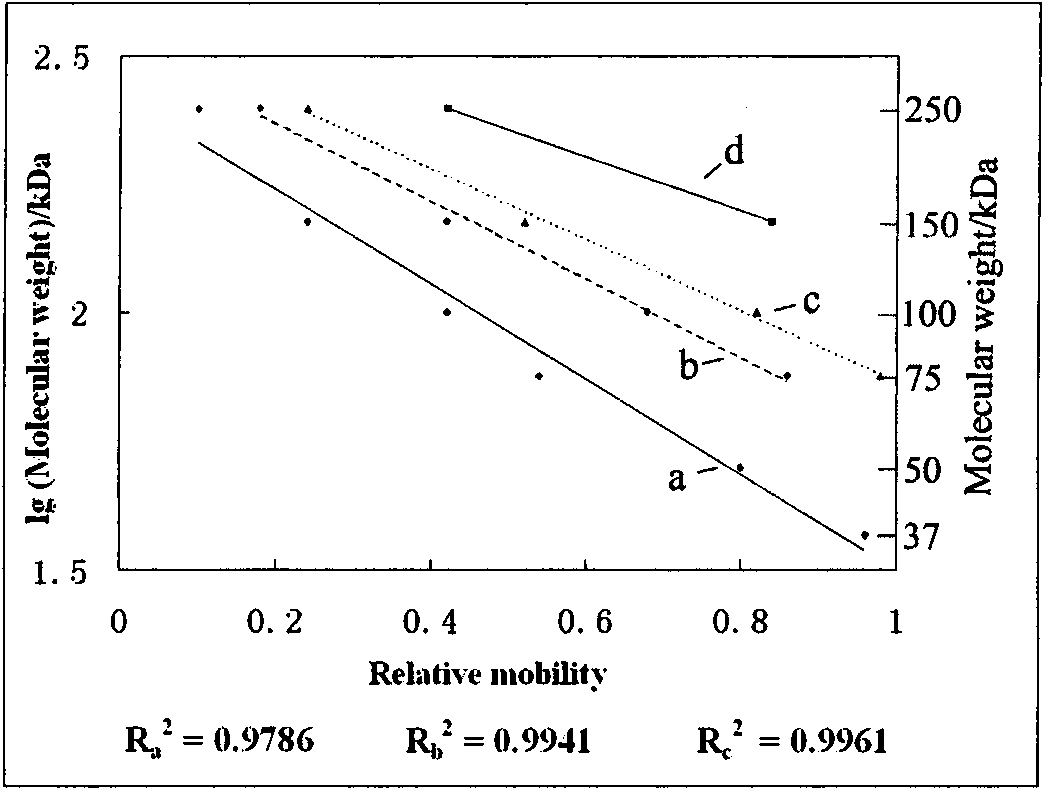
[0034] Standard molecular weight marker proteins (molecular weight range 10-250kDa) were used as standard samples to investigate the separation molecular weight ranges of low melting point agarose and polyacrylamide gel mixtures in different proportions. figure 1 Gel electrophoresis results showing increasing ratio of 0.8% lmpAg from 0 to 1 / 2. As the proportion of lmpAg increases, the average pore size of the hybrid gel increases, and the molecular weight range that can be separated tends to be higher. The separation molecular weight range of each gel can be calculated by Ferguson's law, such as figure 2 Shown: 7% SDS-PAGE, 35-270kDa; SDS-lmpAgPAGE 1 (0.8%SDS-lmpAg / 7%SDS-PAGE gel: 1 / 3, v / v), 60-330kDa; SDS-lmpAgPAGE 2 (0.8 %SDS-lmpAg / 7%SDS-PAGE gel: 1 / 2, v / v), 75-360kDa; SDS-lmpAgPAGE 3 (0.8%SDS-lmpAg / 7%SDS-P...
Embodiment 2
[0035] Example 2 Application example of AgPAGE-LC-ESI-MS / MS route
[0036] The SD-rat brain tissue protein extract purified by GST-Pulldown was used for one-dimensional mixed gel electrophoresis, and compared with the conventional low concentration polyacrylamide gel. image 3 It shows that the use of lmpAgPAGE as the electrophoresis medium makes the separation effect of high molecular weight end significantly better than that of ordinary gel, and the Spectrin isoforms are well separated. The mass spectrometry identification supports this result, see Table 2.
[0037] Table 2 is a list of ESI-MS / MS identification results of contractrin isoforms (Spectrin isoforms). The results showed that the contractile protein isomers were derived from Sprague-Dawley rat (SD-rat) brain purified by glutathione-S-transferase affinity chromatography test (GST-Pulldown). Tissue protein extract. The purified brain tissue protein extract was first separated by low-melting point agarose and polya...
Embodiment 3
[0040] Example 3 Application example of IEF-AgPAGE-MALDI-TOF MS / MS route
[0041] The whole protein of SD-rat large intestine tissue was used for two-dimensional gel electrophoresis. The first dimension used solid-phase gradient Ph dry strip isoelectric focusing, and the second dimension used hybrid gel or ordinary low-concentration polyacrylamide gel electrophoresis. glue, such as Figure 4 shown. ImageMaster 2-D software analysis showed that the number of proteins with a molecular weight higher than 100kDa using the mixed gel was significantly higher than that using the ordinary gel, which were 298 protein spots and 224 protein spots, respectively. For the optional 26 points on the gel, two enrichment methods were used: nano-core-shell complex enrichment and desalination method and centrifugal freeze-drying method. The mass spectrometry data showed that the identification rate of these 26 spots reached 100% by the material enrichment method, while only 7 protein spots were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com