Synthesizing method of porous ion liquid polymer
A technology of ionic liquids and synthesis methods, applied in organic chemistry and other fields, can solve the problems of low adsorption efficiency, long adsorption time, and high technical cost, and achieve the effects of short adsorption time, high CO2 concentration, and low technical cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
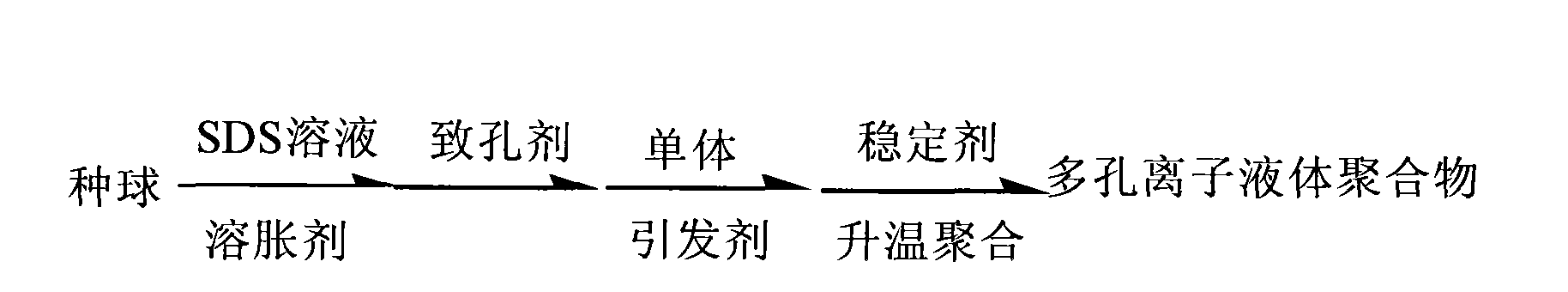
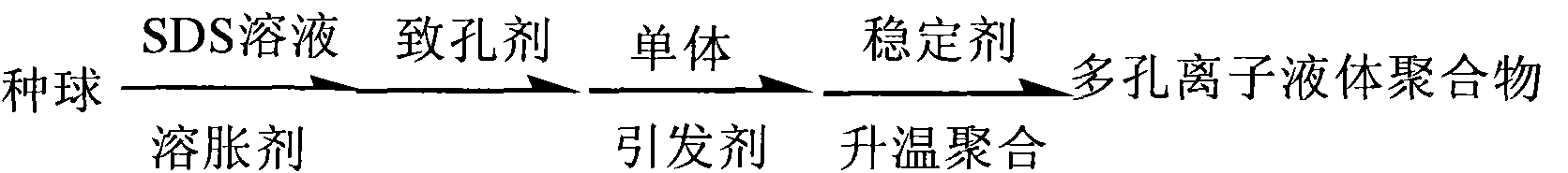
[0018] Embodiment 1: the synthetic process flow that the present invention prepares porous ionic liquid polymer is:
[0019]
[0020] The synthetic method of porous ionic liquid polymer, its synthetic method comprises A, B, C and D four steps:
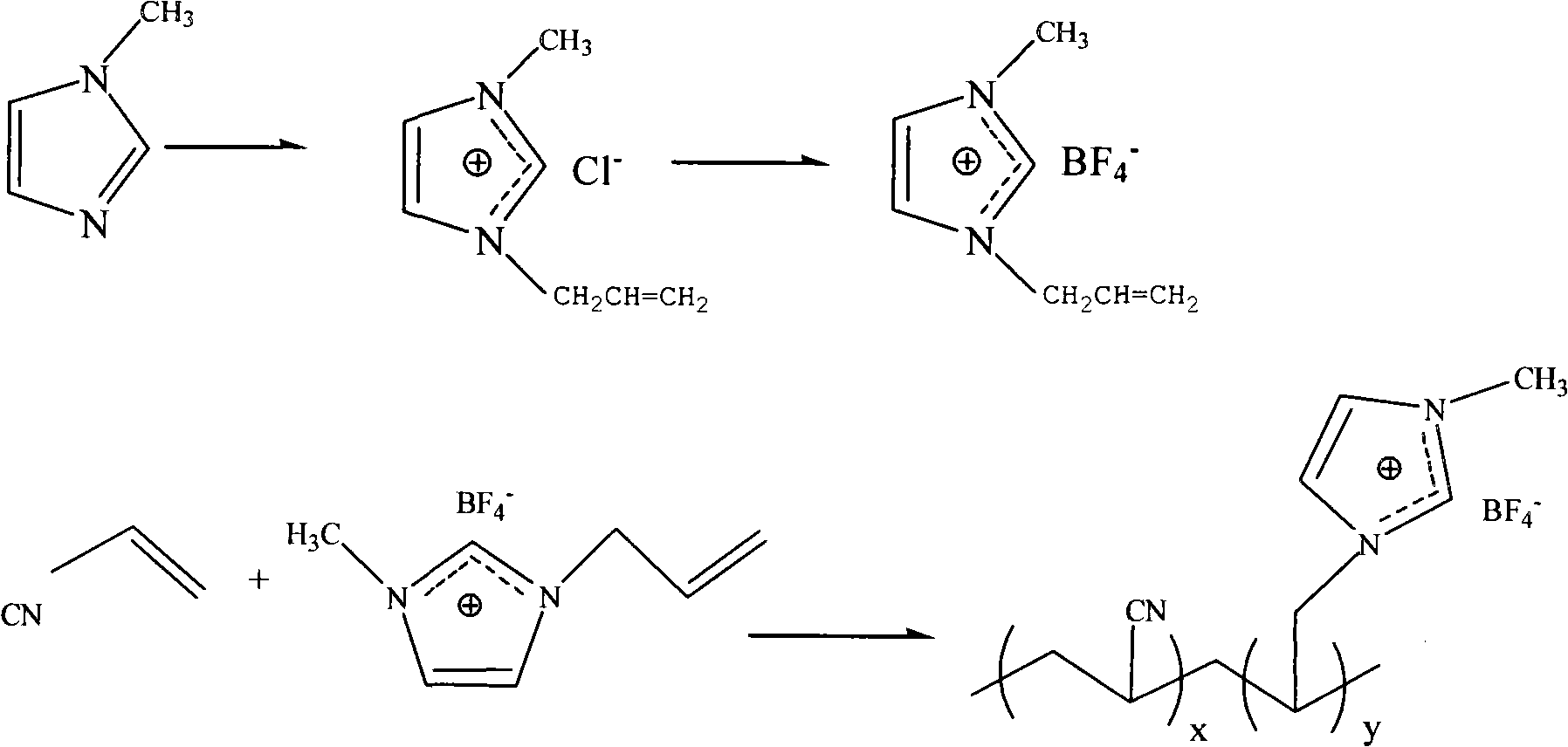
[0021] A. use the chlorinated 1-allyl-3-methylimidazole ionic liquid of formula (II) to react 1-methylimidazole and chloropropene of formula (I); in the synthetic method of preparation formula (II), Under the protection of an inert gas, the molar ratio of 1-methylimidazole and allyl chloride of the formula (I) is 1:1.0 to 1.3, and the reaction is stirred at room temperature to 80°C for 1 to 24 hours;
[0022] B. Use formula (II) to react 1-allyl-3-methylimidazolium ionic liquid and fluoroboric acid to generate 1-allyl-3-methylimidazolium tetrafluoroborate ionic liquid of formula (III) ; In the synthetic method for preparing formula (III), the mol ratio of the chloride 1-allyl-3-methylimidazolium ionic liquid of formula (II) and flu...
Embodiment 2
[0026] Example 2: 1-allyl-3-methylimidazolium tetrafluoroborate ([amim]BF 4 )Synthesis:
[0027] Add 2.0gl-methylimidazole and 2.20g allyl chloride into the three-necked flask, N 2 Protection, heated to reflux in an oil bath at 60°C, and stirred for 7h. Evaporate under vacuum at 40°C to remove excess allyl chloride, and cool to room temperature. Use diethyl ether as the extractant and vigorously stir to remove residual 1-methylimidazole. The solvent was evaporated and dried in vacuo for 4h to obtain dark yellow viscous liquid [amim]Cl.
[0028] 2.51g 40% HBF 4 Slowly added to 2.0g [amim]Cl, stirred electromagnetically at room temperature for 2h, and extracted with chloroform (10mL×3). The organic phase was evaporated in vacuo at 90°C to obtain light yellow mucus [amim]BF 4 .
[0029] 1-allyl-3-methylimidazolium tetrafluoroborate and acrylonitrile copolymer ([amim]BF 4 / AN) synthesis
[0030] 2 mL of anhydrous dimethyl sulfoxide, 0.6 g of acrylonitrile, 1.4 g of [amim]...
Embodiment 3
[0033] Example 3: 10 mL of n-octane was used as the porogen for the two-step seed swelling method, and the rest of the synthesis steps and raw material consumption were the same as in Example 2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com