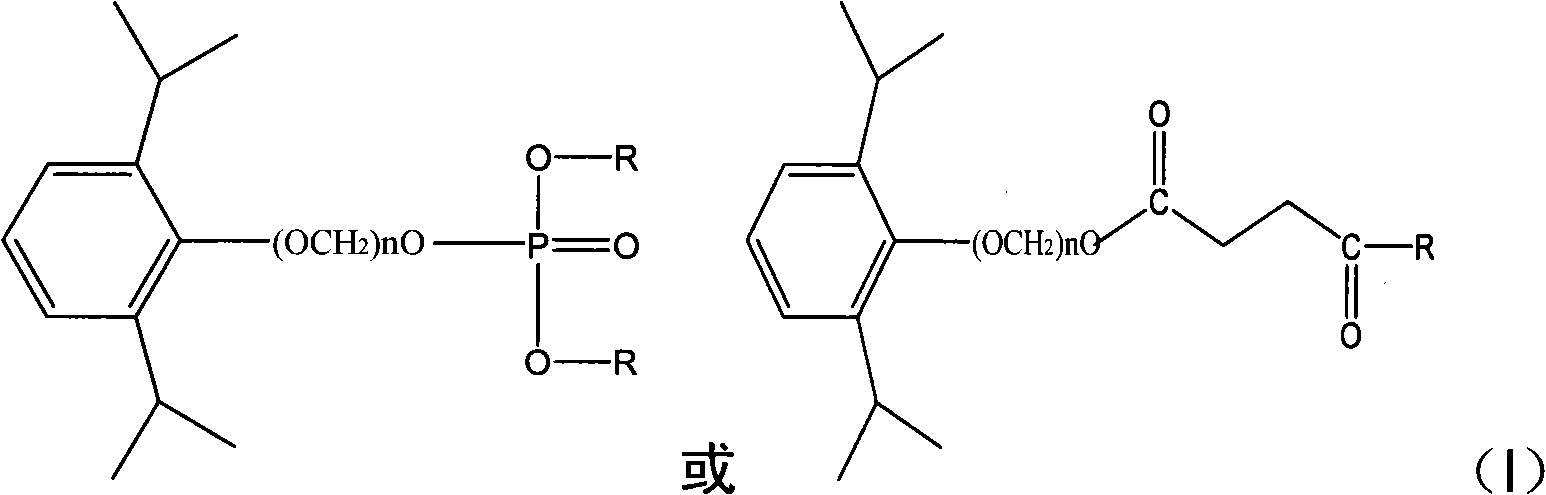
Propofol derivative for therapy
A technology of compounds and hydrates, which is applied to compounds of elements of group 5/15 of the periodic table, drug combinations, phosphorus organic compounds, etc., can solve problems such as low bioavailability and limit treatment development, and achieve good solubility effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
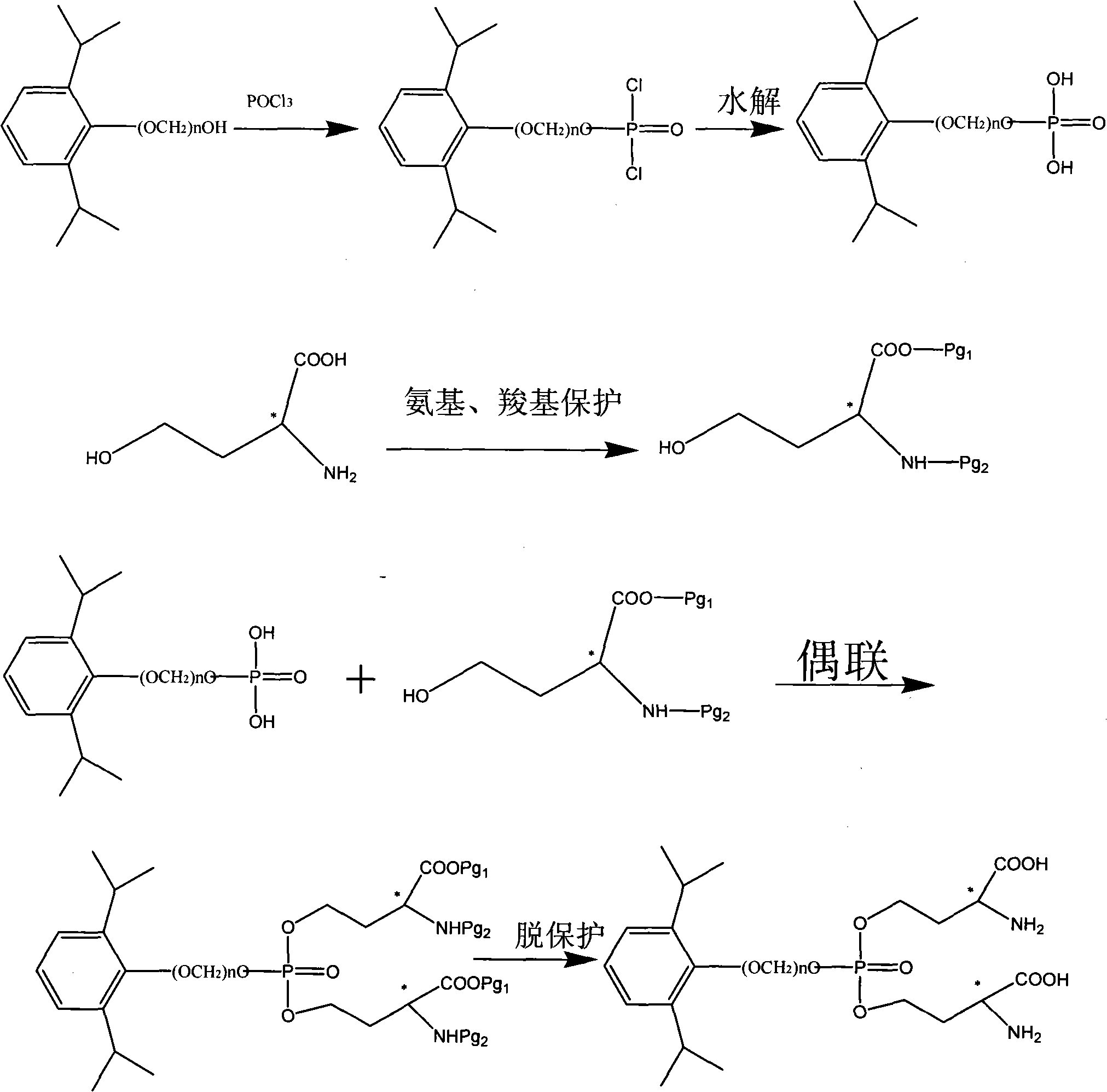
[0048] Embodiment 1: the preparation of compound A
[0049] Dissolve 1.78g of propofol in 20ml of acetonitrile, slowly add 3ml of phosphorus oxychloride solution dropwise at room temperature, after the drop is complete, stir and react at room temperature for 5 hours, slowly add ice water and stir for hydrolysis reaction for 2 hours, evaporate to dryness under reduced pressure Solvent, dissolved in 30ml of tetrahydrofuran, added 3g of serine whose amino group was protected by BOC and carboxyl group by benzyl group, added 2g of triphenylphosphine, slowly added dropwise 2ml of diethyl azodicarboxylate solution, reacted at room temperature for 5 hours, and the reaction was completed , evaporated to dryness under reduced pressure, added ethyl acetate 50ml to dissolve, filtered the insoluble matter, purified by chromatography (eluting with ethyl acetate / n-hexane from n-hexane to 10%) the crude product, combined the filtrates, evaporated to dryness, and then used 30 % trifluoroacetic...
Embodiment 2
[0050] Example 2: Preparation of Compound B.
[0051] It was prepared according to the method of Embodiment 1 except that propofol was replaced by O-(hydroxymethyl)-2,6-diisopropylphenol.
Embodiment 3
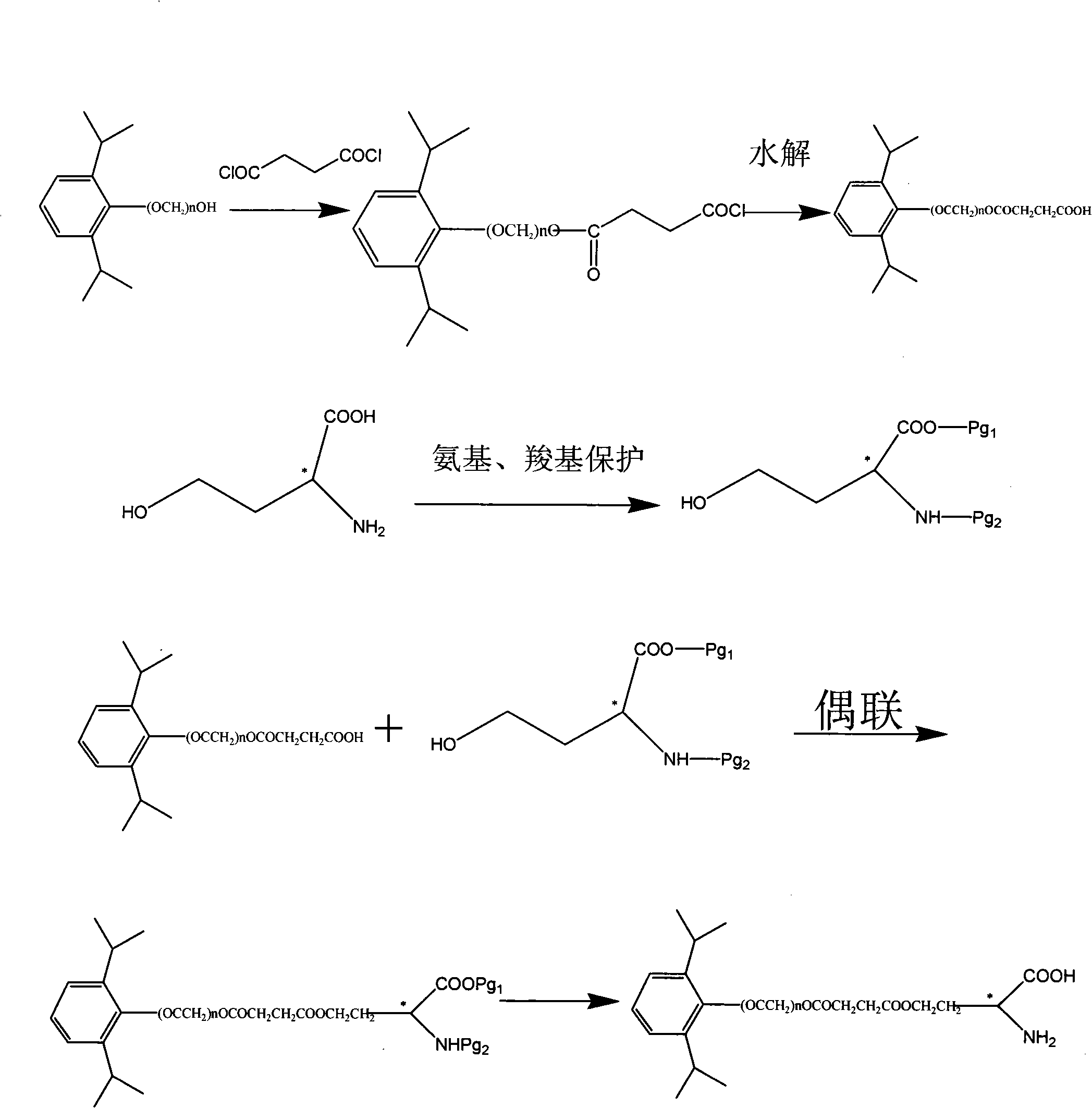
[0052] Example 3: Preparation of Compound C.
[0053] Prepared according to the method of Example 1 except that succinoyl chloride was used instead of phosphorus oxychloride.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com