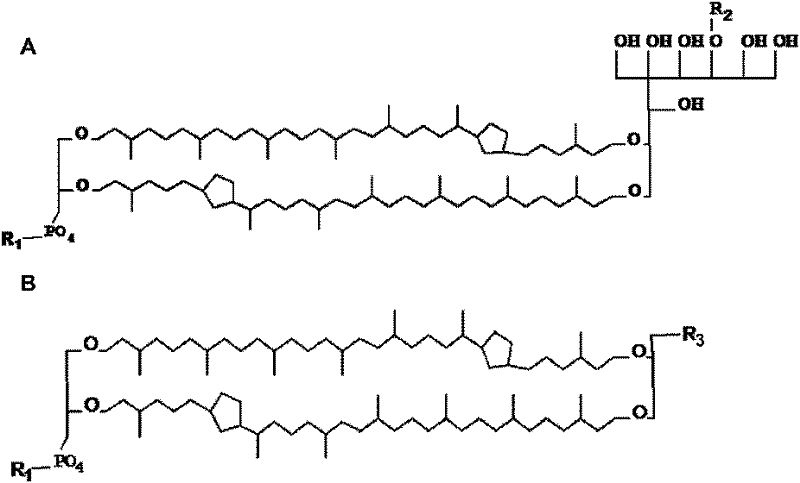
Protein or nucleic acid drug liposome preparation and preparation method thereof based on tetraether lipid
A technology of liposome preparation and nucleic acid drug, which is applied in the directions of liposome delivery, peptide/protein composition, pharmaceutical formulation, etc., can solve the problems of instability of common phospholipid liposomes, and achieves improved bioavailability and protection stability. , improve the efficacy of the drug
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Culturing of Archaea and Extraction and Purification of Tetraether Lipids
[0021] The archaea S. acidocaldarius was inoculated in the ATCC1733 culture solution, pH 2.5, placed in a 65°C bacterial incubator, and the growth of the archaea was monitored by a UV spectrophotometer at 420nm and 540nm respectively. When the archaea grew to logarithmic During the period, it was collected by centrifugation (8800rpm, 10min), and the collected bacterial solution was repeatedly frozen and thawed 3 times at -70-30°C for the extraction of tetraether lipids for later use.
[0022] Suspend the extracted lipids with chloroform: methanol: water = 1:2:0.8 for 12 hours, then add an appropriate amount of chloroform to the suspension to adjust the ratio of chloroform: methanol: water to 2:2:0.8, and continue to suspend After 12 hours, centrifuge (1800rpm, 6min) to obtain an organic phase containing lipids. After filtering to remove impurities, use methanol: water (1:1) to sonicate and apply...
Embodiment 2
[0025] Preparation of blank tetraether liposomes
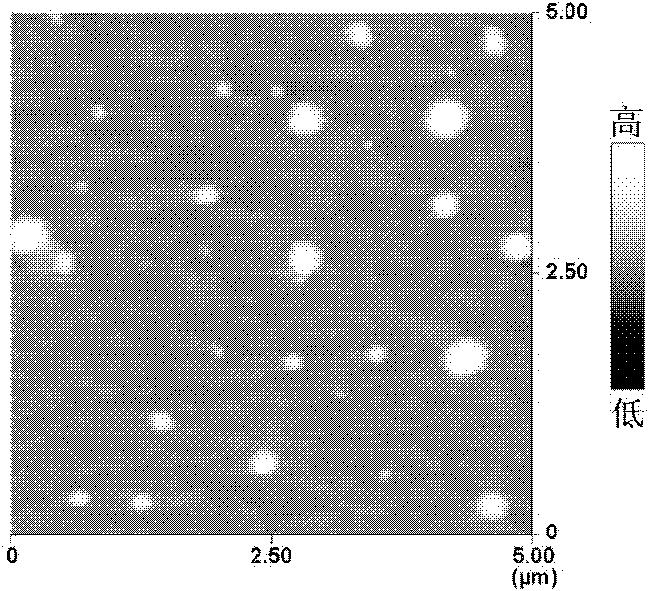
[0026] Take an appropriate amount of PLFE stock solution, add it to a 50mL pear-shaped bottle, then add 6mL chloroform / methanol (2:1) and mix evenly. A uniform and transparent lipid film was formed on the wall of the bottle, and the chloroform and methanol were fully evaporated by filling with nitrogen. According to the appropriate lipid concentration, PBS aqueous solution was added to the pear-shaped bottle for hydration, and the liposomes obtained by ultrasound were measured by Zetasizer 3000HS laser diffraction particle size analyzer (PCS) for particle size distribution, average particle size and Zeta potential. The results showed that the particle size of the blank tetraether liposome was 235.6±12.3nm, the distribution was uniform, the polyindex was less than 0.3, and the surface charge was about -35.1mV.
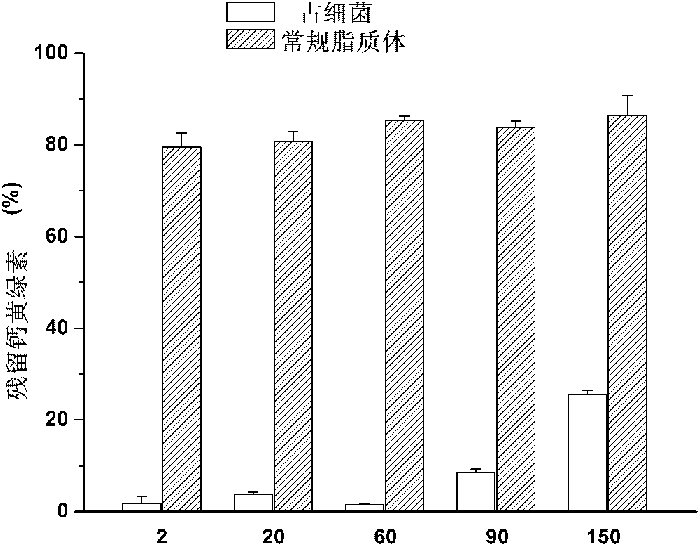
[0027] Stability analysis of tetraether liposomes:
[0028] Using PLFE as the lipid raw material, the liposome part...
Embodiment 3
[0031] Oral administration effect of insulin tetraether liposome preparation
[0032] PLFE is a lipid material, which is hydrated with PBS aqueous solution containing insulin (insulin, 17.5IU / mL) to prepare tetraether liposomes containing protein drugs. The particle size is 268.9±4.8nm, the distribution is uniform, and the polyindex< 0.5, the encapsulation rate is about 20%. 20 diabetic model mice were fasted for 12 hours and randomly divided into 4 groups: the first group was administered intragastrically with insulin solution (insulin, ig) dissolved in phosphate buffer; the second group was intraperitoneally injected with insulin solution (insulin, ip) ; The third group was given conventional liposome-encapsulated insulin solution (Conventional liposomes, ig) by intragastric administration; the fourth group was given intragastric administration of tetraether liposome-encapsulated insulin solution (Archaeosomes, ig). The oral and intraperitoneal insulin concentrations were 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com