Anti-wrinkle composition for external applications to the skin containing biflavonoid derivatives
A technology of flavonoids and derivatives, which is applied in the field of anti-wrinkle compositions for skin external use containing bisflavonoid derivatives, and can solve problems such as instability, skin irritation stability, and skin irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
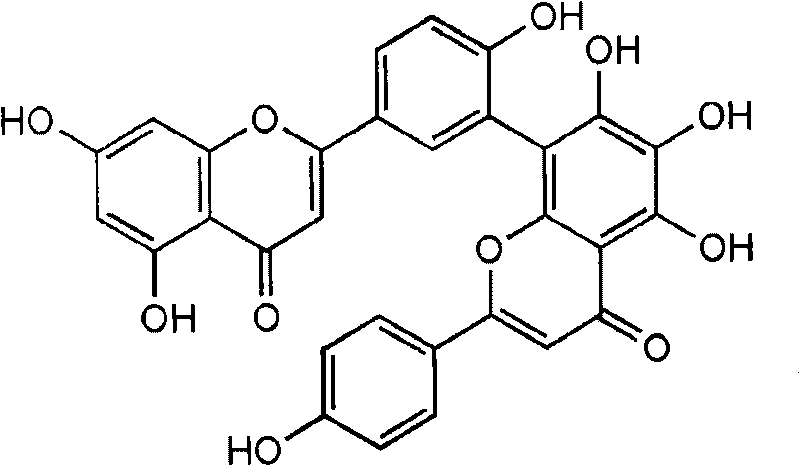
[0059] Embodiment 1: the extraction of sumephedrone
[0060] 1.8 kg of dried Selaginella was cut into small pieces, and then extracted using methanol at 80° C. for 4 hours, and the extraction was repeated three times. The obtained methanol extract was concentrated under reduced pressure. 78.7 g of the concentrate was dissolved in water added with dichloromethane (as an extraction solvent), followed by extraction three times. A dichloromethane-soluble fraction (21.7 g) was obtained, and the residue was extracted three more times using ethyl acetate or butanol as an extraction solvent. As a result, ethyl acetate-soluble components (6.8 g), butanol-soluble components (7.8 g) and residual water components (16.2 g) were obtained.
[0061] Silica gel column chromatography was performed on 6.8 g of ethyl acetate-soluble fraction. A mixed solvent containing chloroform, methanol, and water in a ratio of 10:1:0.1 (vol / vol) was used as a column elution buffer. Column chromatography w...
Embodiment 2
[0063] Embodiment 2: Determination of the structure of Panduratin A compound
[0064] In order to determine the structure of the single material separated in Example 1, measured at 500MHz and 125MHz (solvent: DMSO) respectively 1 H-NMR spectrum and 13 C-NMR spectrum. The result is as follows.
[0065] Will 1 H-NMR and 13 The results of C-NMR were compared with those of traditional studies to identify the structure, and the references are listed below.
[0066] Lin, L.-C., Kuo, Y.-C., and Chou, C.-J. (2000) Cytotoxic biflavonoids from Selaginella delicatula. J. Nat. Prod., 63, 627-630.
[0067] Markham, K.R., Sheppard, C., and Geiger, H. (1987) 13 C NMR studies of some naturally occurring amentoflavone and hinokiflavone biflavonoids. Phytochemistry, 26, 3335-3337.
[0068] Chen, S.-C., Liu, H.-K., and Kuo, Y.-H. (2004) Two new compounds from the leaves of Calocedrus microlepic var. formosana. Chem. Pharm. Bull., 52, 762- 763.
[0069] Chari, V.M., Ilyas, M., Wagner, H....
experiment example 1
[0092] Experimental Example 1: Biosynthesis of Procollagen
[0093] Fibroblasts isolated from human skin were seeded in 24-well plates (10 6 cells / well), followed by culturing until they grow 90%. Then, these cells were cultured in serum-free medium for 24 hours. The 4-substituted benzoic acid derivative compound having 3,4-methylenedioxybenzene or 3,4-ethylenedioxybenzene moiety obtained in Examples 1 to 2 was dissolved in a serum-free medium, and the 10 -4 The concentration of the medium was treated, followed by CO 2 Incubate for 24 hours in the incubator. The supernatant was separated and procollagen levels were determined using a type 1 procollagen ELISA kit. The results are shown in Table 1, and the biosynthetic ability was expressed by the ratio to the untreated group (considered 100%).
[0094] [Table 1]
[0095] compound
[0096] As shown in Table 1, it was confirmed that the biflavonoid derivatives can promote collagen biosynthesis.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com