Dripping pill for treating coronary heart diseases and preparation method thereof
A technique for coronary heart disease and dripping pills, which is applied in the field of pharmaceutical preparations, can solve the problems of poor plasticity, easy sticking of dripping pills, poor stability of dripping pills, etc., and achieves benefits for production and storage, fast dissolution time, and improved compliance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
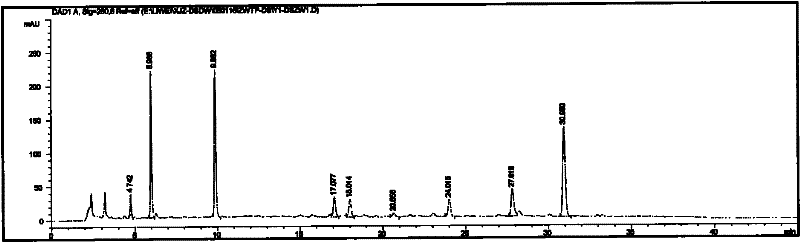
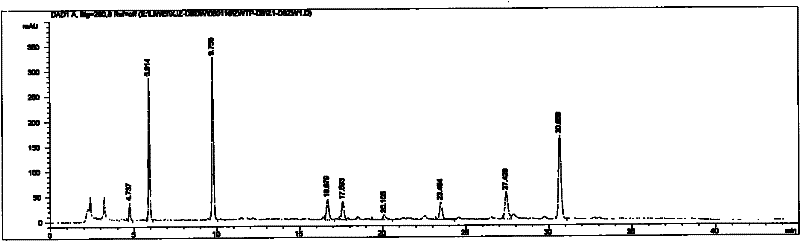
[0069] Extraction Preparation Example The preparation of salvia miltiorrhiza and notoginseng liquid extract (prepared by the method of Chinese patent application CN1348815A embodiment 1)
[0070] Take the coarsely crushed Danshen and Panax notoginseng medicinal materials into the extraction tank, add 5 times the amount of water, decoct for 2 hours, filter, and extract the filter residue for the second time, add 4 times the amount of water, decoct for 1 hour, filter , discard the filter residue, and combine the filtrates. The filtrate is concentrated under reduced pressure to a ratio of 1: (0.9-1.1) to the medicinal liquid volume (L) and the medicinal material weight (Kg), and slowly adds 95% ethanol to make the medicinal liquid contain ethanol concentration at 69-71% (volume percentage). ), stand still for 12 hours, take the supernatant of the medicinal solution after alcohol precipitation, filter, and reclaim ethanol from the filtrate, and concentrate into an extract whose re...
Embodiment 1
[0072] Danshen and Panax notoginseng liquid extract 18g (prepared according to the method of extract preparation example), borneol 1.2g, erythritol 54g, propylene glycol 3g.
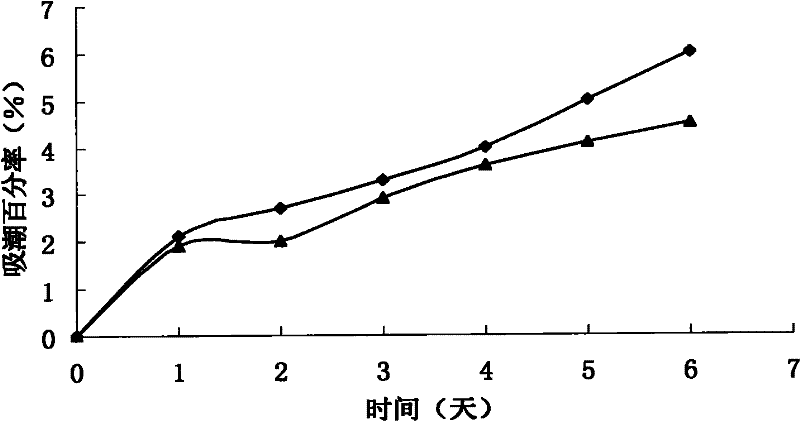
[0073] Thoroughly mix erythritol, salvia miltiorrhiza and Panax notoginseng liquid extract, borneol and propylene glycol, put it into the dripping device, heat it in a circulating oil bath until it melts, keep the temperature of the material at 101°C, and dissolve the melt at 35 drops / Drop into the methyl silicone oil condensate at a temperature of 5°C at a speed of 1 min. After forming, filter the drop pills, and use absorbent paper to absorb the methyl silicone oil adhering to the surface of the drop pills, and dry at low temperature. The results showed that the prepared dropping pills were round, uniform in size, consistent in color and without adhesion. According to the method of disintegration time limit of Chinese Pharmacopoeia 2005 edition, the results show that the average 1.96min without baffle...
Embodiment 2
[0075] Salvia miltiorrhiza and Panax notoginseng dry extract (fluid extract prepared according to the method of extract preparation example, obtained by conventional spray drying) 19.2g, borneol 4.8g, erythritol 32.7g, propylene glycol 2.4g, water 0.9g.
[0076] Fully mix erythritol, salvia miltiorrhiza and Panax notoginseng dry extract, borneol, propylene glycol and water, put it into the dripping device, heat it in a circulating oil bath until it melts, keep the temperature of the material at 105°C, and melt the melt at 40°C Drop into the liquid paraffin condensate at a temperature of 7°C at a rate of drops / min. After forming, filter the drop pills, use absorbent paper to absorb the liquid paraffin adhering to the surface of the drop pills, and dry at low temperature. The results showed that the prepared dropping pills were round, uniform in size, consistent in color and without adhesion. According to the method of disintegration time limit of Chinese Pharmacopoeia 2005 edit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com