Nitrilase gene, vector, engineering bacteria and application thereof
A technology of genetically engineered bacteria and nitrilase, which is applied in the field of preparation of recombinant nitrilase, can solve problems such as no breakthrough of nitrilase, low efficiency, and difficulty in selecting site-directed mutation sites
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The total genomic DNA of Arthrobacter nitroguajacolicus CCTCC No: M 208252 was extracted with a rapid nucleic acid extraction instrument, and the genomic DNA was used as a template to carry out PCR amplification under the action of primer 1 (ATGGATCACCCGAAATTCAAAGC) and primer 2 (AATACCTTCTTGGTCAGTCGGC) . The amount of each component in the PCR reaction system (total volume 50 μL): 5 μL of 10×Pfu DNA Polymerase Buffer, 1 μL of 10 mM dNTP mixture (2.5 mM each of dATP, dCTP, dGTP, and dTTP), 1 μL of cloning primer 1 and primer 2 at a concentration of 50 μM , genomic DNA 1 μL, Pfu DNA Polymerase 1 μL, nucleic acid-free water 40 μL.
[0056] Using Biorad’s PCR instrument, the PCR reaction conditions are: pre-denaturation at 94°C for 5 minutes, then enter the temperature cycle at 94°C for 30s, 53°C for 1min, and 72°C for 1.5min, a total of 30 cycles, and finally extend at 72°C for 10min, and the termination temperature is 8°C .
[0057] Take 5 μL of the PCR reaction soluti...
Embodiment 2
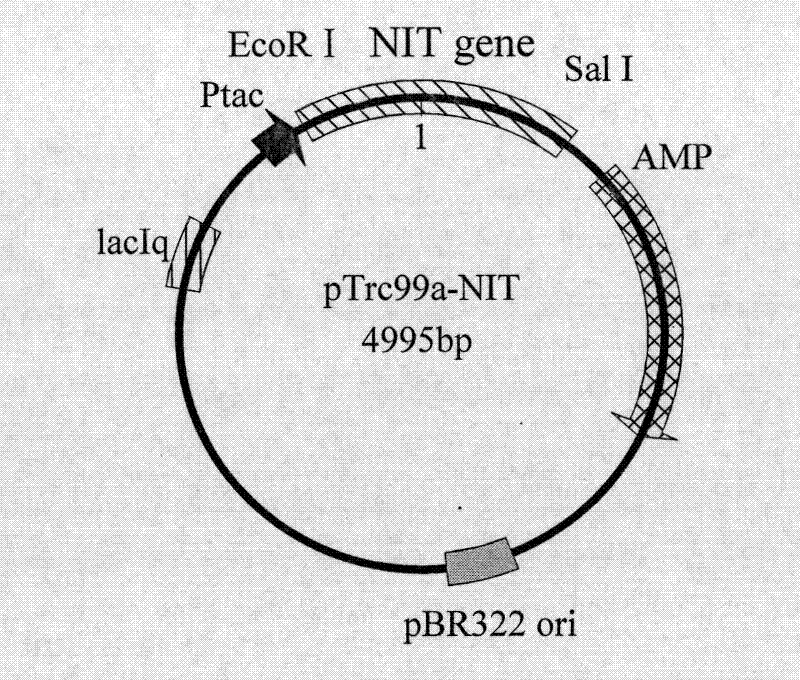
[0059] According to embodiment 1 analysis result design primer 3 (cgc gaattc atggatcacccgaaattcaaagcagc), primer 4 (gtt gtcgac ttaaataccttcttggtcagtcggcag), and introduced EcoRI and SalI restriction enzyme sites in primer 3 and primer 4, respectively. Under the priming of primer 3 and primer 4, high-fidelity Pyrobest DNA polymerase (TaKaRa) was used to amplify to obtain a 819bp nitrilase gene fragment (its nucleotide sequence is shown in SEQ ID NO: 1), After sequencing, the amplified fragment was treated with EcoRI and Sal restriction enzymes (TaKaRa), and the fragment was combined with the commercial vector pTrc99a (Invitrogen ) were connected to construct the expression vector pTrc99a-NIT. The constructed intracellular expression vector pTrc99a-NIT was electrotransformed into Escherichia coli JM109 (Invitrogen), plated and cultured at 37°C overnight, and clones were randomly selected to extract plasmids for enzyme digestion identification. The identification results are s...
Embodiment 3
[0061] According to embodiment 1 analysis result design primer 5 (cgc gaattc gatcacccgaaattcaaagcagc), primer 6 (att gtcgac aataccttcttggtcagtcggcag), and introduced EcoRI and SalI restriction enzyme sites in primer 5 and primer 6, respectively. Under the priming of primer 5 and primer 6, high-fidelity Pyrobest DNA polymerase (TaKaRa) was used to amplify to obtain a 819bp nitrilase gene fragment, which was sequenced using EcoRI and SalI restriction endonuclease (TaKaRa) The amplified fragment was processed, and the fragment was ligated with the commercialized vector pET20b treated with the same restriction endonuclease using T4 DNA ligase (TaKaRa) to construct the secreted expression vector pET20b-NIT. The constructed expression vector pET20b-NIT was electrotransformed into Escherichia coli BL21 (Invitrogen), plated and cultured overnight at 37°C, clones were randomly picked and plasmids were extracted for enzyme digestion identification. The identification results are shown...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com