schistosomiasis japonica arborization carrier-DNA vaccine and preparation method thereof
A DNA vaccine and schistosomiasis technology, applied in recombinant DNA technology, DNA / RNA fragments, pharmaceutical formulations, etc., can solve the problems of short retention and expression, difficulty in treatment and prevention, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Characteristic Analysis of Dendritic Vector-DNA Vaccine
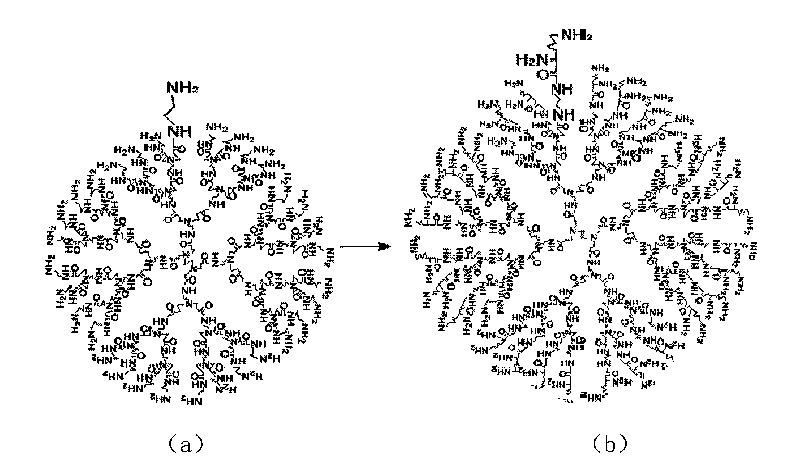
[0048] 1. Electrophoretic retardation experiment: Determine the optimal charge ratio R for complete complexing of plasmid DNA and PAMAM-Lys dendrimer by agarose gel electrophoresis + / - . The results showed that PAMAM-Lys had the strongest ability to bind DNA when the charge ratio was 4.
[0049] 2. Morphological investigation of the dendrimer-DNA vaccine: the vaccine morphology was observed with a transmission electron microscope (TEM). Drop 10 μL of plasmid DNA and dendritic carrier-DNA vaccine solution on the copper grid respectively, and absorb the solvent for 2 minutes, and blot the solvent dry with filter paper; Blot dry with filter paper. After drying, place it under the electron microscope for observation. Results After the plasmid DNA was combined with the dendritic vector, the diameter of the pure plasmid became smaller, and the particle diameter of the dendritic vector-DNA vaccine tended ...
Embodiment 4
[0059] Example 4 Detection of cytokines in splenocyte culture supernatant
[0060] Three weeks after the last immunization, 2 mice in each group were killed by pulling the neck, and the spleen was aseptically taken out, and a single spleen cell suspension was prepared by conventional methods, and SjC23-large hydrophilic fragment protein (SjC23-HD) [Schistosome strain) 23kD molecule (SjC23) large hydrophilic peptide (HD) was expressed in PGEX-5X-1. Yu Chuanxin. Zhu Yinchang. Yin Xuren. He Wei. Hua Wanquan. Journal of Practical Parasitic Diseases 2000 (4)] as a stimulus to induce cytokines. Specific steps:
[0061] 1) Kill the mice by pulling their necks, soak in 75% alcohol for 3-5 minutes, take them out and place them in a plate, and take the spleen aseptically.
[0062] 2) The spleen was ground with tweezers, filtered through a 200-mesh stainless steel mesh, and suspended to 5 mL with incomplete RPMI-1640 (GIBCO) culture medium.
[0063] 3) Centrifuge at 1000rpm for 5min; ...
Embodiment 5
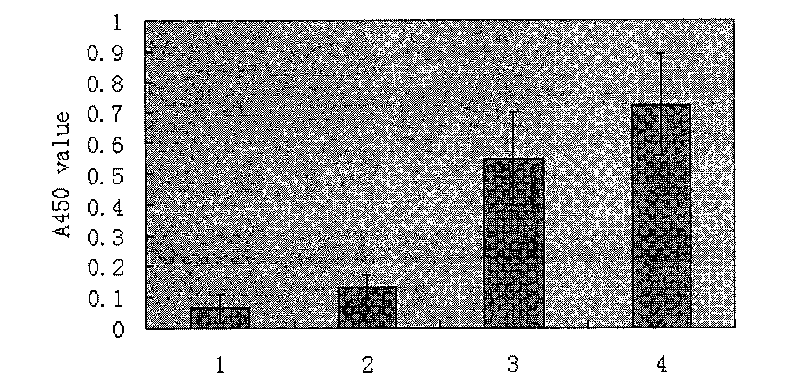
[0070] Example 5 Immunoprotective Test
[0071] Fifty experimental mice were randomly divided into 4 groups, namely pJW4303 (control group 1), PAMAM-Lys (control group 2), pJW4303-SjC23 and PAMAM-Lys / pJW4303-SjC23, with 12-14 mice in each group. The mice in each group were injected with pJW4303, PAMAM-Lys, pJW4303-SjC23 and PAMAM-Lys / pJW4303-SjC23 into the quadriceps femoris, 100 μg per mouse, 50 μg on the left and right sides. Booster immunization once every 2 weeks, with the same dose and method, immunization 3 times in total. Four weeks after the third immunization, each mouse was infected with (40±1) cercariae through the abdominal skin. Mice were dissected 42 days after challenge infection, flushed with citrate saline, and adult worms were collected from the portal vein and counted. The liver was taken out, weighed, cut into pieces, placed in 5 mL of 5% KOH solution, digested overnight at 37°C, and eggs were counted. Calculate the insect reduction rate and egg reduction...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com