Dry powder inhalation, preparation method and application thereof
A dry powder inhaler, powder technology, applied in the field of pharmaceutical preparations, can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] Prescription: Hydroxycamptothecin 50mg main drug
[0063] Lactose 5g Excipients
[0064] Leucine 0.5g Excipients
[0065] Ammonium carbonate 23g Foaming agent
[0066]Sodium hydroxide solution Appropriate amount pH adjuster
[0067] Distilled water 300mL solvent
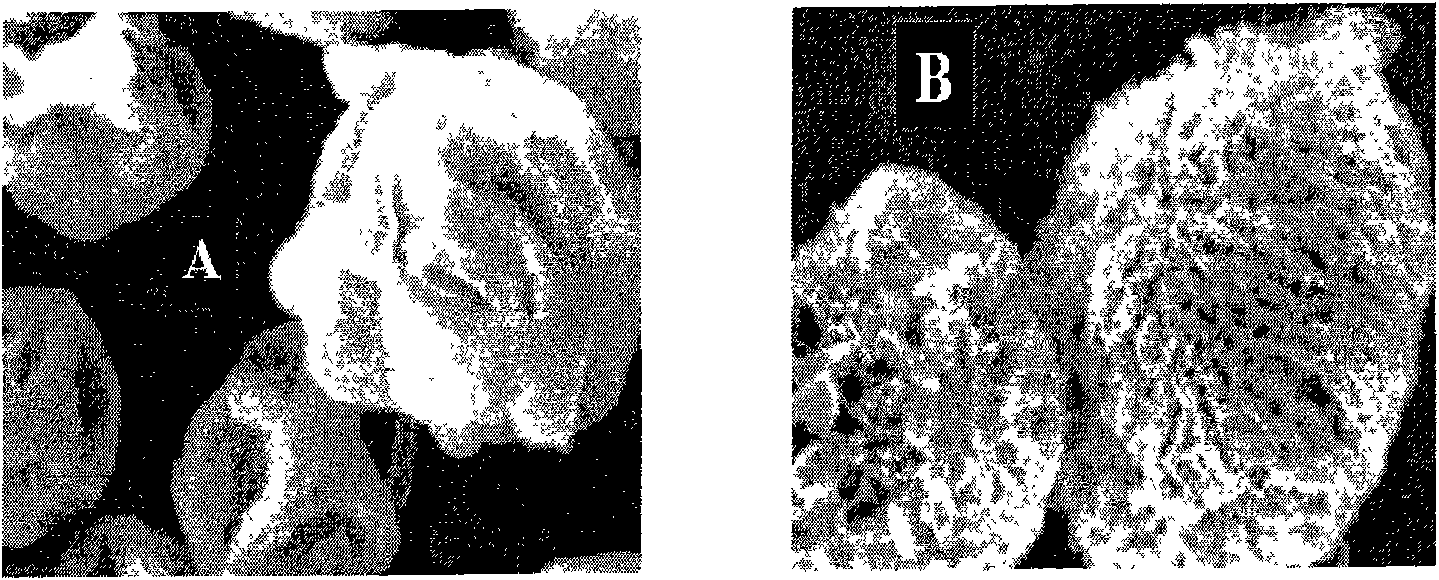
[0068] Add the above-mentioned main ingredients, auxiliary materials and foaming agent into distilled water, stir to nearly dissolve, and adjust the pH to 10.5 with sodium hydroxide solution. Spray drying according to the following conditions: inlet temperature 130°C, liquid spray speed 3mL / min, hot air volume 90%, atomizing air flow rate 700L / hour, and prescription B was obtained. Repeat the above experiment without adding ammonium carbonate to obtain prescription A as a control.
[0069] Scanning electron micrographs of the spray-dried particles are shown in figure 1 A and figure 1 b. The results show that the addition of ammonium carbonate has a great influence on the appearance of the spray-dried p...
Embodiment 2
[0074] Prescription: recombinant human interferon α-2b (potency 1.6×10 8 IU / mg) main drug
[0075] Lactose Excipients
[0076] Lysine Excipients
[0077] Leucine Excipients
[0078] ammonium carbonate blowing agent
[0079] pH 7.0 Citric Acid-Disodium Hydrogen Phosphate Solvent
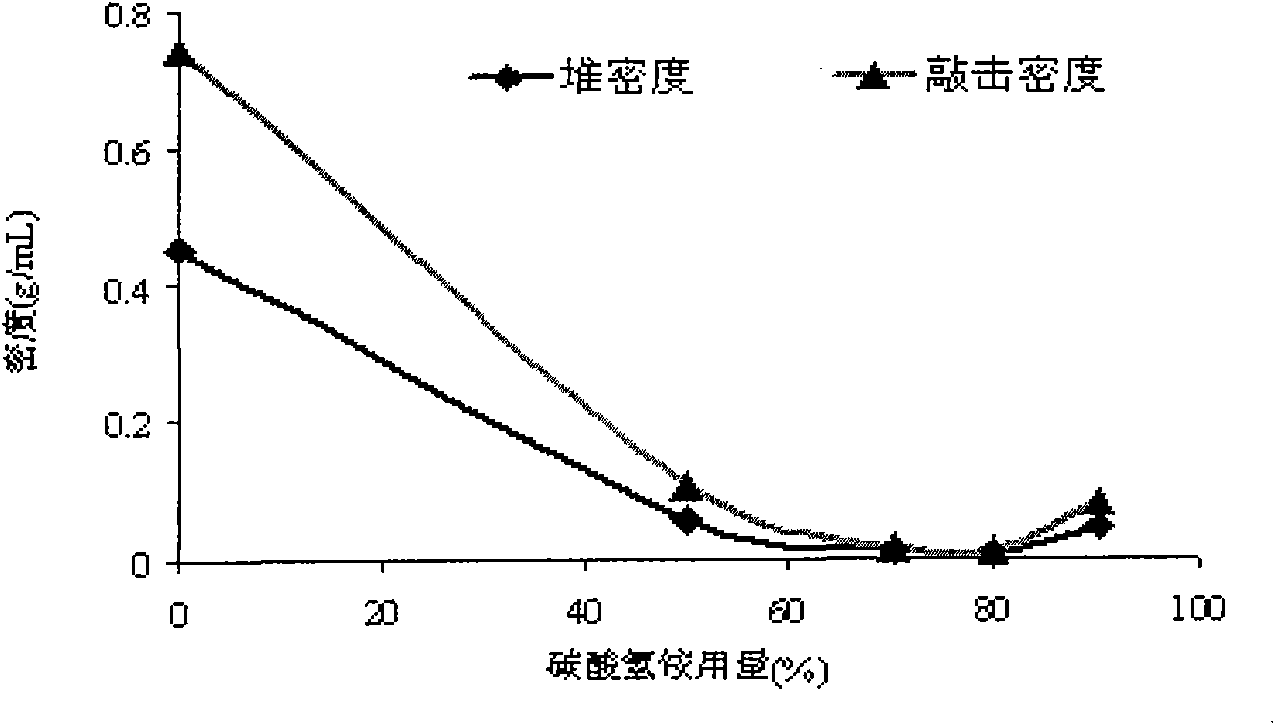
[0080] An appropriate amount of lactose was dissolved in a citric acid-disodium hydrogen phosphate buffer system with pH 7.0 to make a lactose solution with a concentration of 1.4%, and a small amount of lysine was added as an active protective agent for interferon and an appropriate amount of leucine (to make it The concentration is 6% of the solute content) as a dispersion aid. Add an appropriate amount of recombinant human interferon α-2b to make the concentration of interferon 1.12×10 6 IU / mL, finally add the ammonium bicarbonate of series concentration, make the content of ammonium bicarbonate be respectively 0%, 50%, 70%, 80%, 90% of solute content, spray dry according to the following con...
Embodiment 3
[0086] Prescription: Etoposide liposome main drug, solvent
[0087] Mannitol Excipients
[0088] Leucine Excipients
[0089] ammonium carbonate blowing agent
[0090] Take 0.2g of etoposide, 1.2g of soybean lecithin and 0.4g of cholesterol, dissolve in 25mL of chloroform, and remove the chloroform by rotary evaporation in a water bath at 30°C to obtain the etoposide-phospholipid complex; another 150mL of pH7.4 phosphate buffer solution was added to Into the above complex, ultrasonic treatment for 30 minutes, to obtain etoposide liposomes. Add 1g mannitol, 0.5g leucine and 7g ammonium carbonate to the liposome solution, spray dry according to the following conditions: inlet temperature 105°C, spray speed 3mL / min, hot air volume 90%, atomizing air flow rate 700L / Hour. The above experiment was repeated without adding ammonium carbonate as a control.
[0091] The deposition results showed that the deposition rate of the formulation without ammonium carbonate was only 11.2%, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bulk density | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Bulk density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com