An anti-hcv vaccine and preparation methods and uses thereof
A vaccine and purpose technology, applied in the field of genetic engineering, can solve the problems of damage to DC, narrow scope of applicable population, weak immune response, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
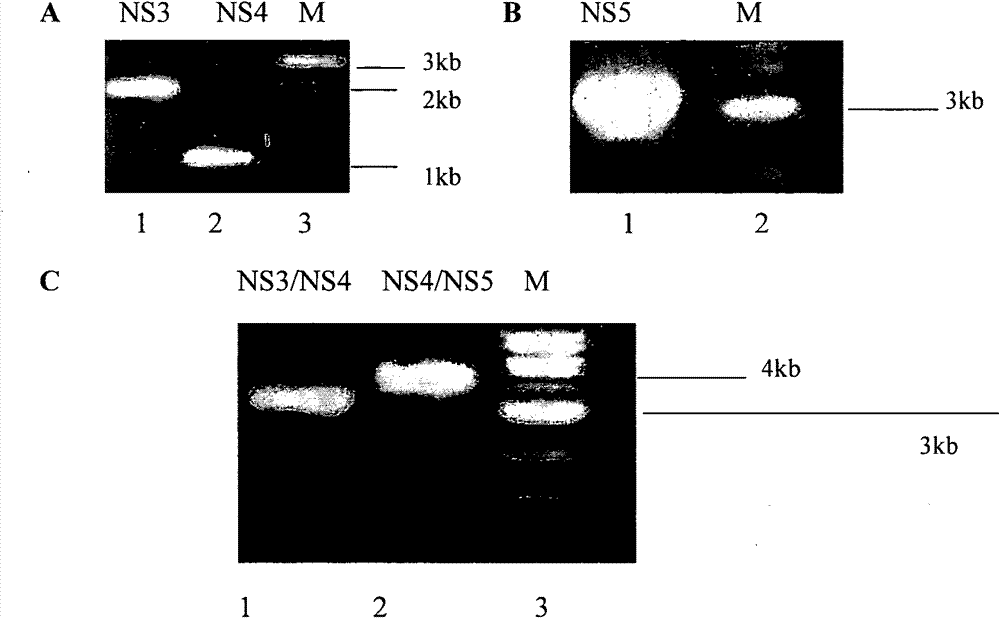
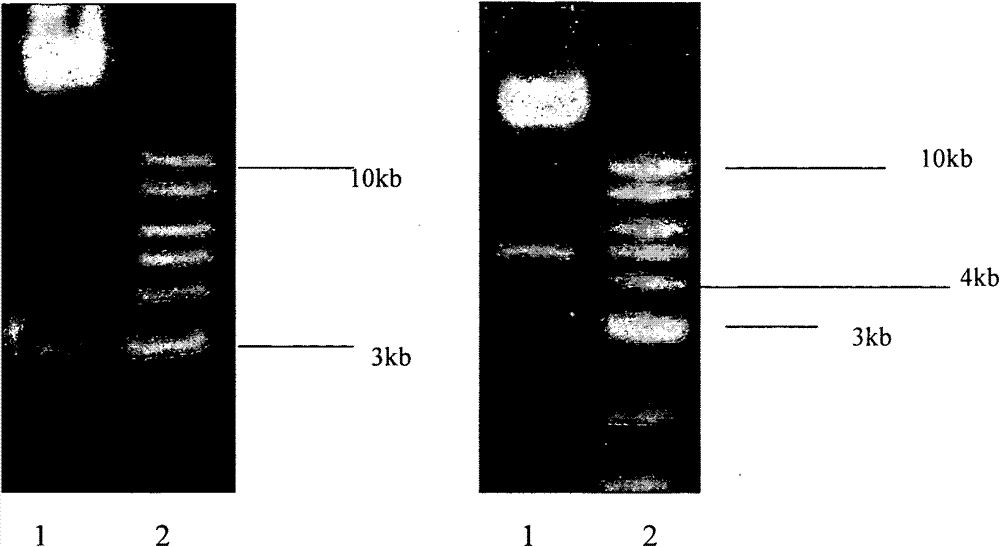
[0032] Example 1: Construction of Adenoviral Vectors Carrying Various Nonstructural Genes of HCV and Expression Identification of HCV Proteins
[0033] 1. Experimental materials
[0034] Sera from HCV-infected patients were obtained from voluntary donors with signed informed consent.
[0035] Pshuttle-CMV (Germany, Merck, Cat. No. ST240007), Adeasy-1 plasmid (Germany, Merck, Cat. No. ST240005), Escherichia coli BJ5183 and recombinant adenovirus carrying GFP (AdGFP) were purchased from Beijing Nuosai Genome Research Center limited company;
[0036] Huh7 liver cancer cell line was purchased from People's Hospital (Jia Yintang et al., Construction of eukaryotic expression vector of interferon-stimulated gene ISG20 and its anti-hepatitis C virus replication, Chinese Journal of Immunology, 2006Vol.22No.11P.997-1001) ;
[0037] DH5α chemical transformation competence was purchased from Beijing Dingguo Biotechnology Co., Ltd.;
[0038] M-MLV Rtase cDNA synthesis Kit and LA-Taq DN...
Embodiment 2
[0121] Embodiment 2: healthy human body experiment in vitro
[0122] 1. Experimental materials
[0123] 1) Research object: The peripheral blood of 19 HLA-A2 positive healthy people was obtained from Beijing Red Cross Blood Center.
[0124] 2) Lymphocyte stratification fluid: purchased from Dingguo Biotechnology Co., Ltd., specific gravity 1.077g / ml; recombinant human granulocyte-macrophage colony-stimulating factor (rh GM-CSF) and recombinant human interleukin 4 (rh-IL -4) Purchased from an American R&D company;
[0125] AIM-V serum-free medium was purchased from GIBCO, USA;
[0126] RPMI 1640 medium was purchased from Dingguo Biotechnology Co., Ltd. Complete RPMI 1640 contained 10% fetal bovine serum, 1% glutamine, 100IU / ml streptomycin and 100μg / ml penicillin;
[0127] Mouse anti-human HLA-A2 monoclonal antibody was purchased from BD Company in the United States, and LPS and mitomycin were purchased from Sigma Company in the United States;
[0128] Hhu7 cells (HCVR) sta...
Embodiment 3
[0170] Embodiment 3: In vitro experiment of HCV infected persons
[0171] 1. Experimental materials
[0172] Cit
[0173] 2. Experimental method
[0174] The treatment method of the HCV infection group was the same as that of the D group in Example 2. When non-adherent PBMCs interacted with DCs, they first interacted for 24 hours, added IL-230IU / ml, replaced half of the medium every other day, and continued to cultivate for 48 hours. Others are the same as before.
[0175] 3. Experimental results (see Table 7)
[0176] Adenoviral vectors carrying HCVNS3 / NS4 and HCVNS4 / NS5 genes respectively induced strong cellular immune responses, but there was no statistical difference between them, and both were stronger than the immune responses induced by DCs loaded with 7 peptides.
[0177] Table 7HCV infection group IFN-γ, IL-4 and GrB ELISPOT results (SFC / 2×10 5 PBMC)
[0178]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com