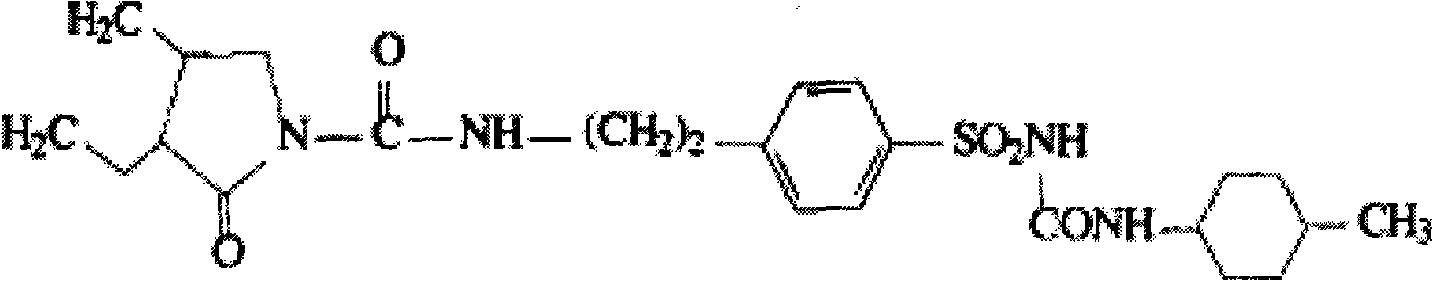
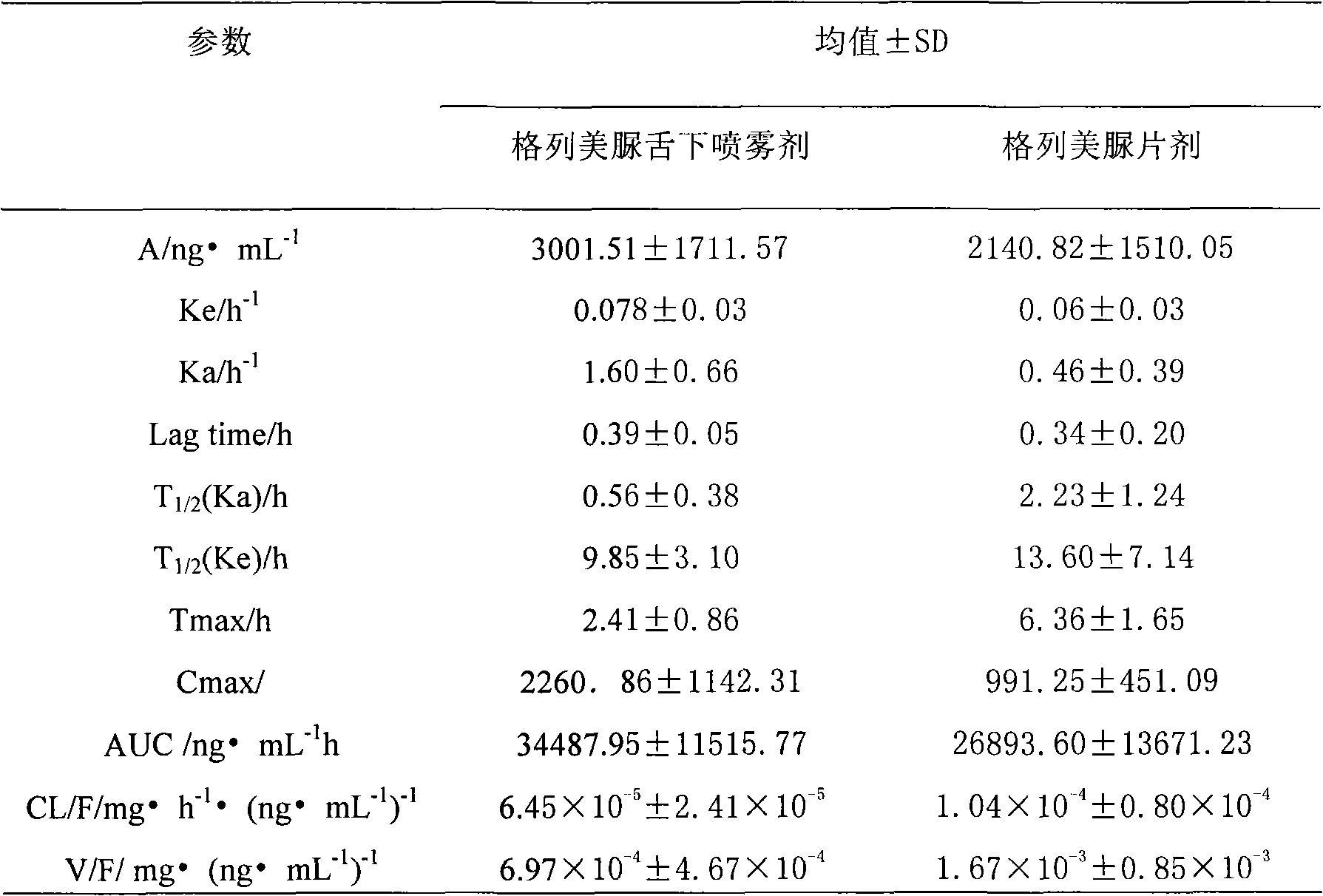
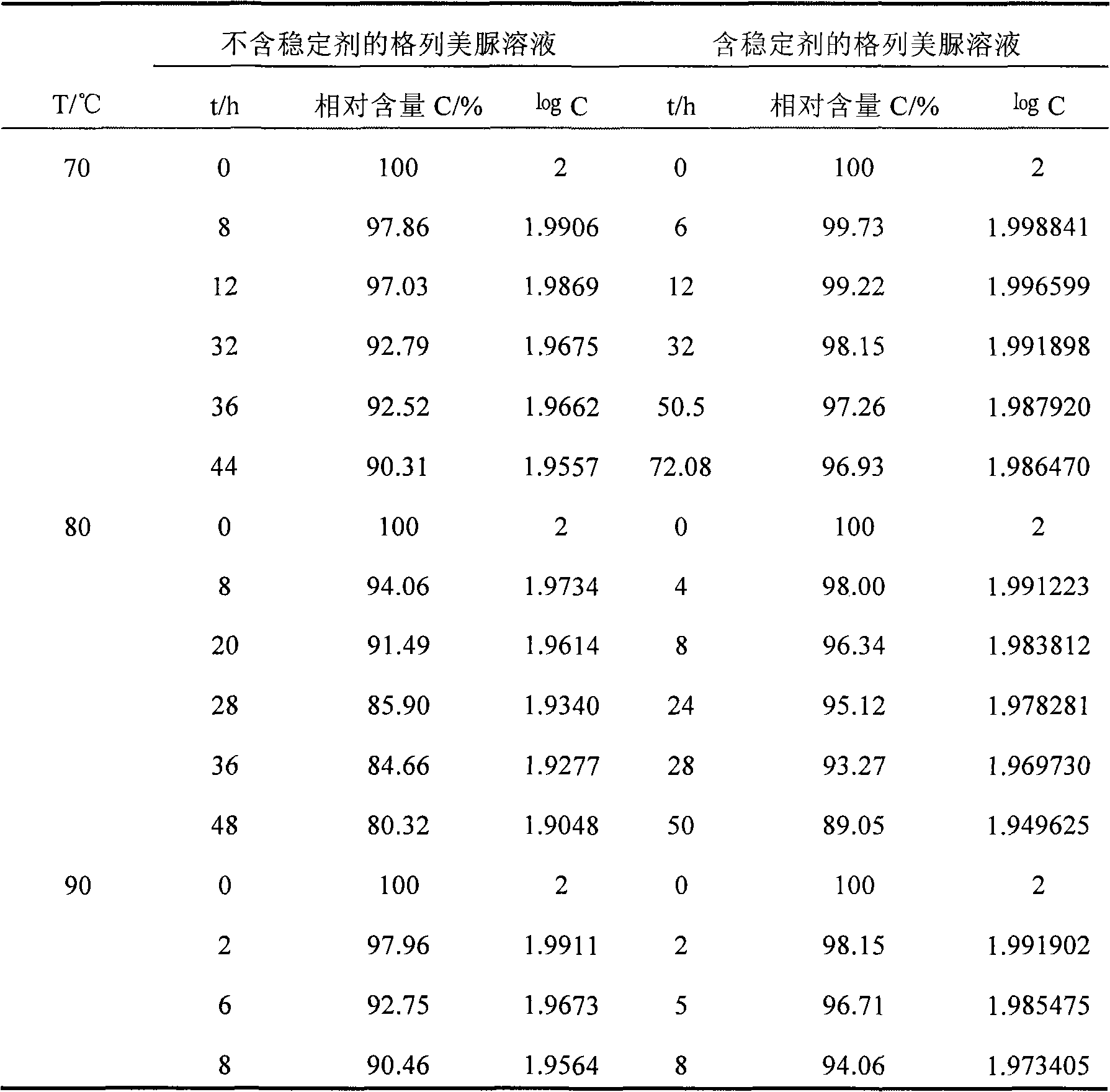
Glimepiride aqueous solution administration system and preparation method thereof
A glimepiride and aqueous solution technology, applied in the field of medicine, can solve the problems of poor stability, low dissolution rate and bioavailability of a glimepiride solid oral preparation, achieve simple and feasible preparation process, avoid liver first-pass effect, Avoid the effects of digestive tract effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1: Glimepiride Sublingual Spray
[0041] 1. Components:
[0042] Glimepiride 10g
[0043] Tween 80 6g
[0044] Meglumine 45g
[0045] Poloxamer 188 2g
[0046] Hydroxypropyl-β-cyclodextrin 1g
[0047] Glycerin 200ml
[0048] Steviol Glycosides 0.4g
[0049] Double distilled water to 1000ml
[0050] 2. Preparation method:
[0051] First pass the glimepiride and meglumine through a 100-mesh sieve, mix the glimepiride and the prescribed amount of meglumine first, add an appropriate amount of water, and after ultrasonication for about 20 minutes, heat and dissolve in a water bath at 70±10°C until clear After the solution, add Tween 80, poloxamer 188, glycerin, hydroxypropyl-β-cyclodextrin, steviol glycoside in turn respectively in the prescribed amount, add an appropriate amount of redistilled water, and after ultrasonic mixing, add water to 1000ml, Then filter, can, seal, pack, and pass the inspection to be the finished product.
Embodiment 2
[0052] Example 2: Glimepiride Sublingual Spray
[0053] 1. Components:
[0054] Glimepiride 10g
[0055] Meglumine 25g
[0056] Tween 80 6g
[0057] Hydroxypropyl-β-cyclodextrin 1g
[0058] Glycerin 200ml
[0059] Aspartame 0.4g
[0060] Double distilled water to 1000ml
[0061] 2. Preparation method:
[0062] First pass the glimepiride and meglumine through a 100-mesh sieve, mix the glimepiride and the prescribed amount of meglumine first, add an appropriate amount of water, and after ultrasonication for about 20 minutes, heat and dissolve in a water bath at 70±10°C until clear After the solution, add the prescribed amount of Tween 80, glycerin, hydroxypropyl-β-cyclodextrin, and aspartame in turn, add an appropriate amount of double-distilled water, and after ultrasonic mixing, add water to 1000ml, then filter and pack , sealing, packaging, and inspection is the finished product.
Embodiment 3
[0063] Example 3: Glimepiride Sublingual Spray
[0064] 1. Components:
[0065] Glimepiride 10g
[0066] Meglumine 50g
[0067] Tween 80 10g
[0068] Hydroxypropyl-β-cyclodextrin 1g
[0069] Ethanol 200ml
[0070] Steviol Glycosides 1g
[0071] Double distilled water to 1000ml
[0072] 2. Preparation method:
[0073] First pass the glimepiride and meglumine through a 100-mesh sieve, mix the glimepiride and the prescribed amount of meglumine first, add an appropriate amount of water, and after ultrasonication for about 20 minutes, heat and dissolve in a water bath at 70±10°C until clear After the solution, add Tween 80, ethanol, hydroxypropyl-β-cyclodextrin, and steviol glycoside in turn in the prescribed amount, add an appropriate amount of double-distilled water, mix evenly with ultrasound, add water to 1000ml, then filter, can, Sealing, packaging, and passing the inspection is the finished product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com