Herba epimedii aglycone or cyclo-herba epimedii aglycone phospholipid compound and preparation method thereof
A technology for cyclic icariin and astrogenin phospholipids, which is applied in the field of pharmaceutical preparations, can solve the problems of poor water solubility, fat solubility, and low bioavailability, achieve high drug loading, simple preparation process, and overcome loading The effect of low dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
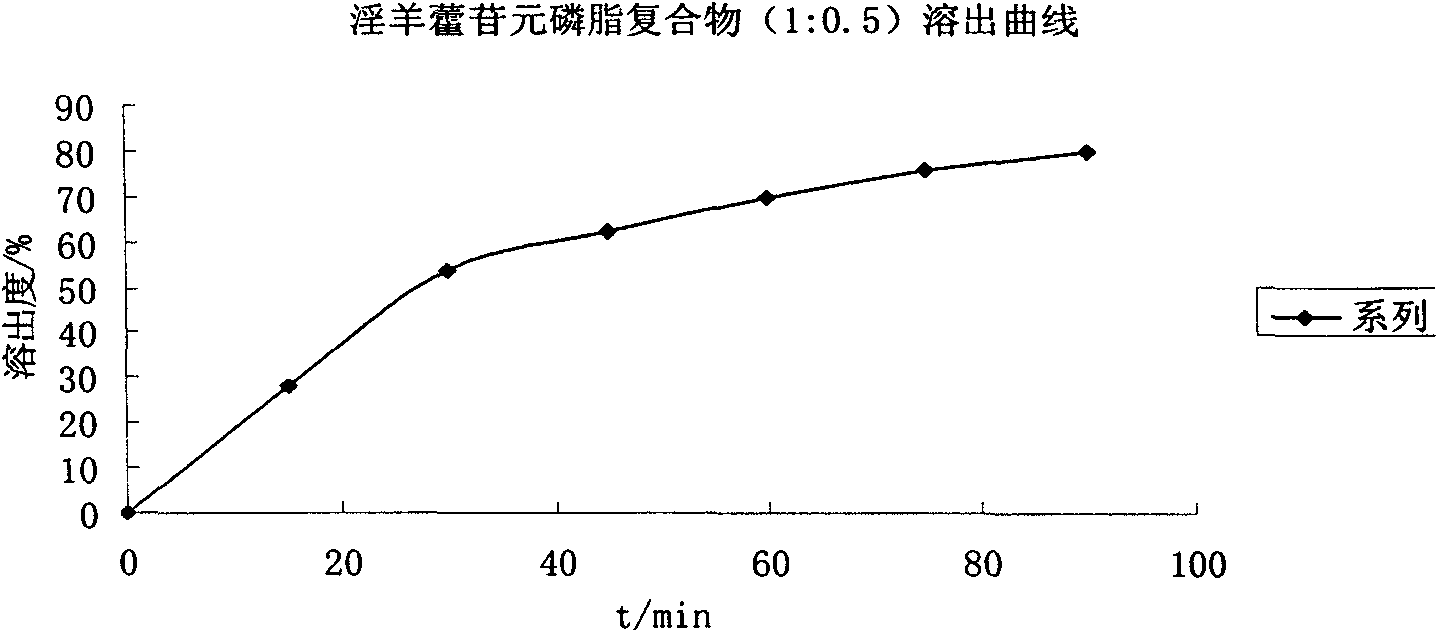
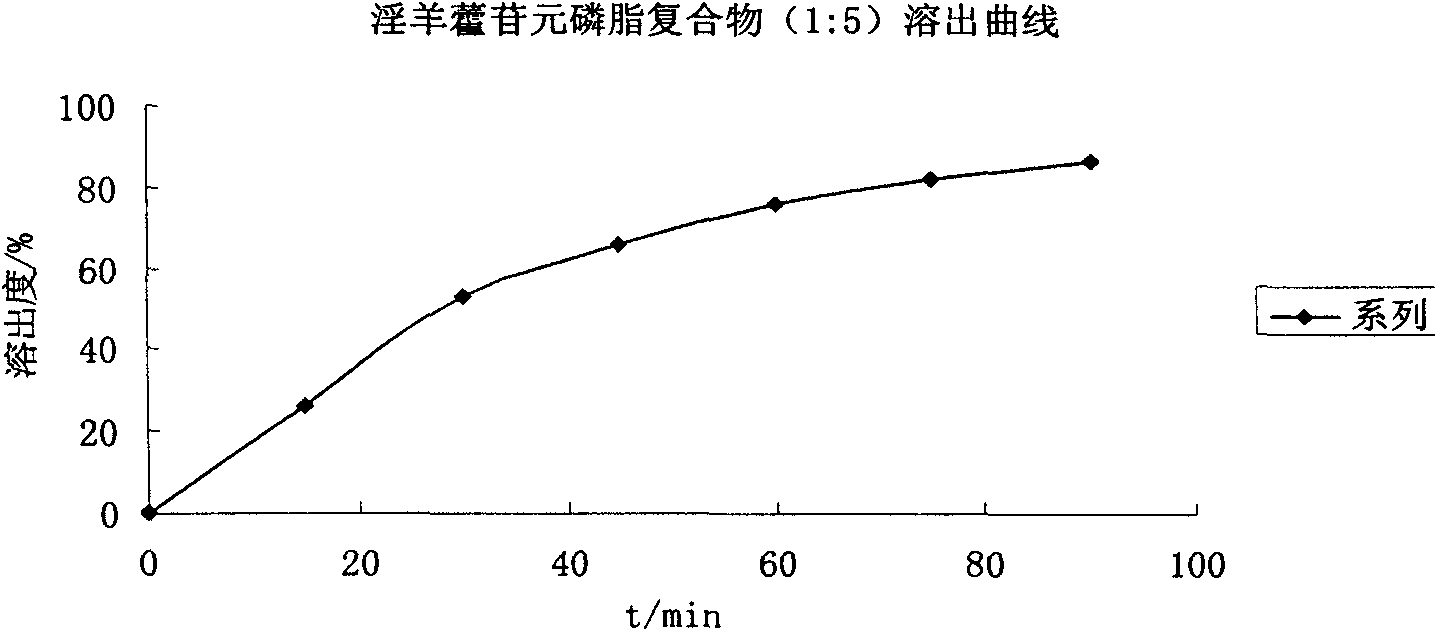
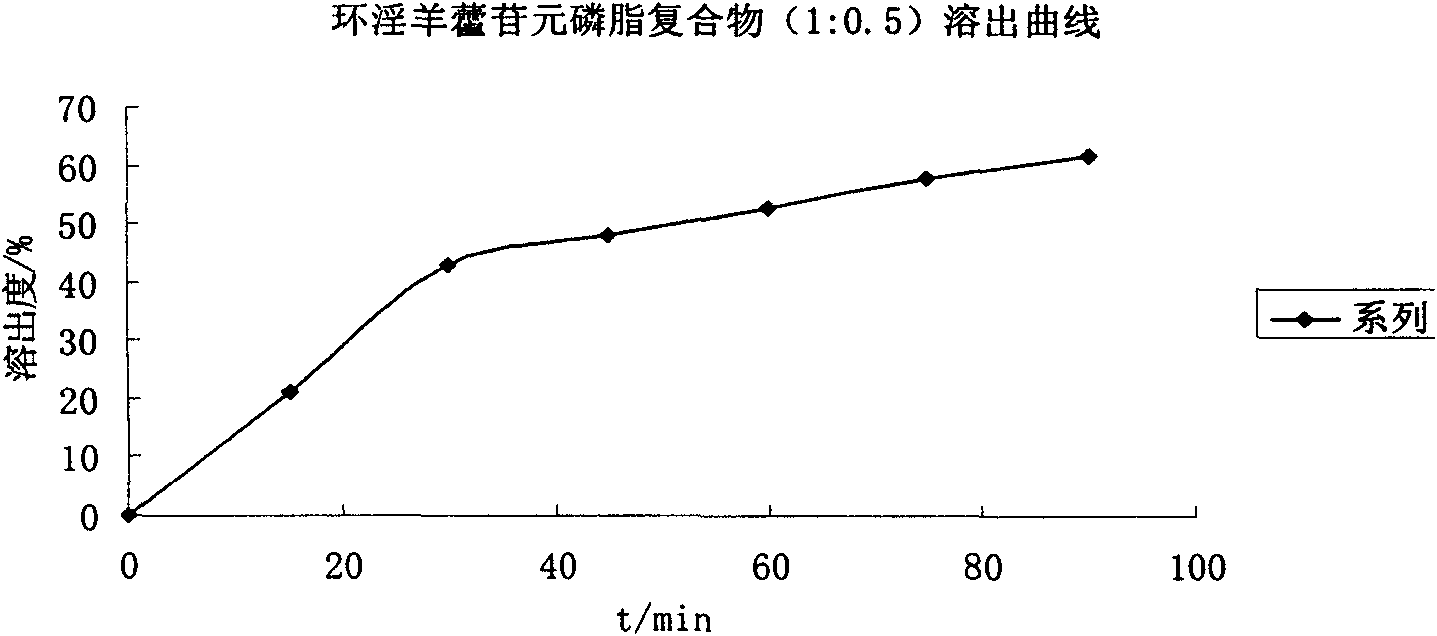
[0053] Example 1. In vitro dissolution test of icarigenin, cycloicarigenin raw materials and their phospholipid complexes
[0054] Take icariin or cycloicariin raw material drug and soybean lecithin, their mass ratio is 1:0.5 or 1:5 plus 50 times the mass of absolute ethanol, heat and reflux for 1 hour, the yellow Slightly sticky powder, conduct equilibrium solubility and in vitro dissolution tests.
[0055] Equilibrium solubility experiment:
[0056] Three batches of prepared mixtures and complexes of icariin, cycloicariin and soybean phospholipid were weighed according to the drug / phospholipid mass ratio of 1:0.5 and 1:5 respectively, and the measured average equilibrium solubility is shown in the table below.
[0057] Equilibrium solubility table of icariin, cyclic icariin and soybean lecithin mixture and complex
[0058] ethanol
water
n-octanol
IT
5.30mg / mL
insoluble
4.77mg / mL
IT / phospholipid (1:0.5 mixture
9.51mg / mL
...
Embodiment 2
[0066] Example 2. Preparation of icarigenin phospholipid complex
[0067] Weigh 20g of icariin, 40g of soybean lecithin, add 500mL of ethanol, reflux at 50°C for 1h, until the reaction solution is clear, evaporate the solvent, and dry the solid in vacuum to obtain a yellow solid, which is crushed through an 80-mesh sieve to obtain Epimedium Huogenin phospholipid complex, the resulting product has no new absorption peaks after ultraviolet spectrum scanning, see Figure 6 ; Infrared spectrum see Figure 10 , the results showed that the infrared spectrum analysis spectrum of the icariin phospholipid complex was not consistent with the infrared spectrum analysis spectrum of the mixture. The infrared spectrum of the physical mixture basically retains the characteristic absorption peaks of the drug and phospholipids. It is a simple superposition of the infrared chromatographic peaks of icariin and phospholipids. There is no interaction between the two, and icariin remains in cryst...
Embodiment 3
[0068] Example 3. Preparation of Icarigenin Phospholipid Complex
[0069] Weigh 20g of icariin, 10g of lecithin, add 500mL of methanol, stir at 15°C for 6h until the reaction solution is clear, evaporate the solvent, and dry the solid in vacuum to obtain a yellow solid, which is crushed through a 80-mesh sieve to obtain Epimedium Aurigenin phospholipid complex, its ultraviolet scanning spectrum is the same as implementation example 2, see Figure 13 . Take 30 g of the prepared phospholipid complex, add 40 g of microcrystalline cellulose, 30 g of starch, and 5 g of sodium carboxymethyl starch, wet granulate with 95% ethanol as a wetting agent, dry, add 0.5 g of magnesium stearate, and press into tablets , to get 1000 tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com