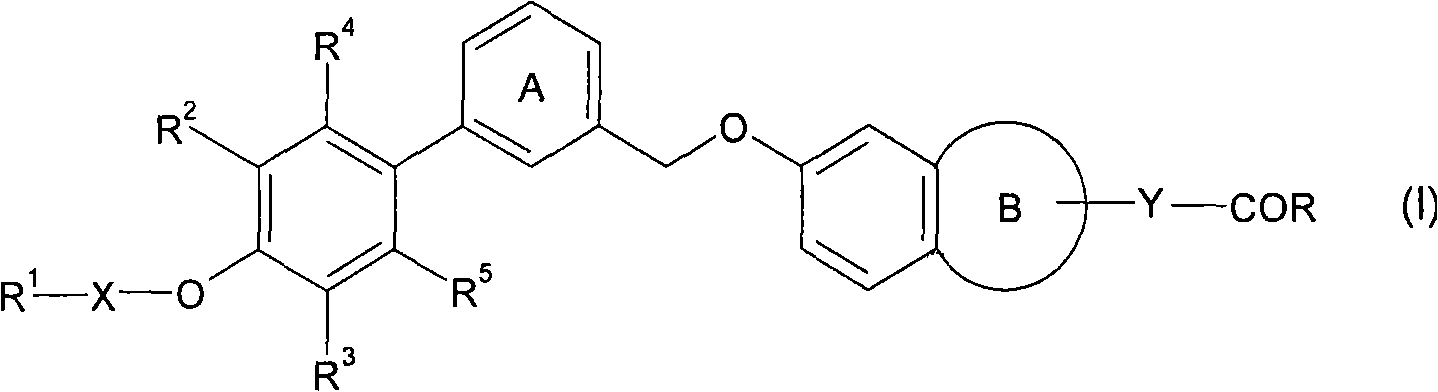
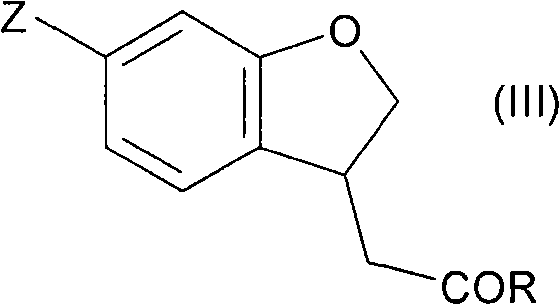
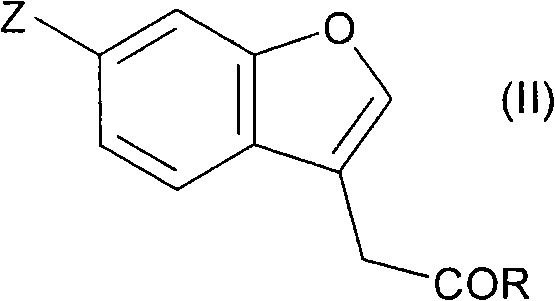
Fused cyclic compounds
A compound, benzene ring technology, applied in the digestive system, organic chemistry, drug combination, etc., can solve the problem of undisclosed compounds, etc., and achieve the effects of excellent stability, good pharmacokinetic properties, and excellent GPR40 receptor agonist activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0353] The production method of compound (I) is explained below.
[0354] Unless otherwise stated, the individual symbols of the compounds in the following schemes are as defined above. Each compound in the scheme can form a salt as long as it does not inhibit the reaction. As such salts, those similar to the salts of compound (I) can be mentioned.
[0355] The compound obtained in each step can also be used in the next reaction as a crude product in the form of a reaction mixture, or can be separated from the reaction mixture according to a conventional method, and can be further easily purified by separation methods such as recrystallization, distillation, chromatography, etc. .
[0356] Compound (I) (for example, compounds represented by formulas (Ia) and (Ia') (abbreviated as compound (Ia) and compound (Ia', respectively))) can be obtained according to the method shown in Scheme 1 below or a similar method Prepare.
[0357] Diagram 1
[0358]
[0359] where R 1 ' ...
Embodiment
[0560] The present invention is further described in detail by the following reference examples, examples, formulation examples and experimental examples, which are merely examples, not intended to limit the present invention, and can be changed without departing from the scope of the present invention.
[0561] The term "room temperature" in the following Reference Examples and Examples means generally a range of about 10°C to about 35°C. The chemical yield (yield) is the isolated yield (mol / mol%) or the yield obtained by high performance liquid chromatography. The optical purity (asymmetric yield) of the optically active form is calculated on the basis of enantiomeric excess (% e.e.). The enantiomeric excess is determined by the following formula:
[0562] Enantiomeric excess (%e.e.) = 100X[(R)-(S)] / [(R)+(S)] or
[0563] 100X[(S)-(R)] / [(R)+(S)]
[0564] where (R) and (S) are the areas of the individual enantiomers in HPLC.
[0565] Solvents used in chromatography are exp...
reference example 1
[0596] Reference Example 1 4-(4-bromo-3,5-dimethylphenoxy)tetrahydro-2H-thiopyran
[0597]
[0598]To 4-bromo-3,5-dimethylphenol (0.201g, 1.00mmol), tetrahydro-2H-thiopyran-4-ol (0.130g, 1.10mmol) and triphenylphosphine (0.341g, 1.30mmol ) in tetrahydrofuran (5 mL) was added diethyl azodicarboxylate (40% solution in toluene, 0.591 mL, 1.30 mmol), and the mixture was stirred at room temperature for 1.5 hours. Add tetrahydro-2H-thiopyran-4-ol (0.0591 g, 0.500 mmol), triphenylphosphine (0.157 g, 0.600 mmol) and diethyl azodicarboxylate (40% solution in toluene, 0.272 mL , 0.600 mmol), the mixture was further stirred for 1.5 hours. The reaction mixture was concentrated under reduced pressure, and the residue was purified by silica gel column chromatography (ethyl acetate:hexane=0:100-20:80) to obtain the title compound (0.261 g, yield 86%) as colorless crystals.
[0599] 1 H NMR (CDCl 3 )δ: 1.93-2.07 (2H, m), 2.10-2.23 (2H, m), 2.37 (6H, s), 2.49-2.61 (2H, m), 2.85-2.98 (2H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com