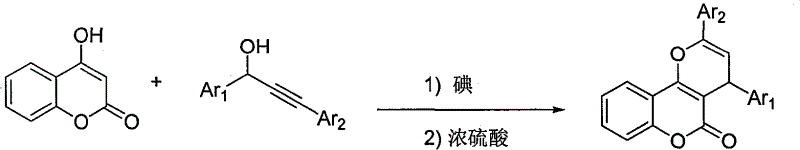
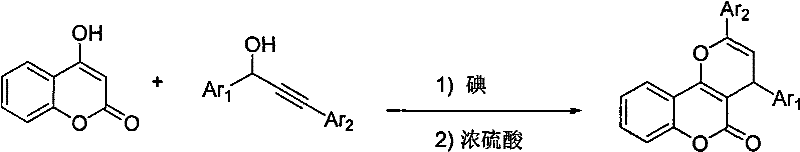
Method for synthesizing pyrano-coumarin derivative
The technology of a coumarin derivative and a synthesis method is applied in the field of synthesis of coumarin derivatives, can solve the problems of expensive reagents, complicated process operation and the like, and achieves the effects of low cost, simple operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] 4-Hydroxycoumarin (10 mmol) and 1,3-diphenyl-2-propyn-1-ol (10 mmol) were dissolved in nitromethane and catalyzed by molecular iodine (1.0 mmol) React at 50°C for 1 hour, add 0.5 ml of sulfuric acid dropwise, stir overnight at 80°C, add 20 ml of saturated sodium sulfite solution, extract with 20×2 ml of ethyl acetate, dry the organic phase over anhydrous sodium sulfate, and concentrate under reduced pressure , and then purified by column chromatography to obtain 3,4-(2,4-diphenylpyrano)coumarin with a yield of 65%. The physical data of the product are: white solid; mp: 173-175°C; IR (KBr): 1716, 1631, 1610, 1493, 1454, 1387, 1271, 1205, 1169, 1112, 1013, 765, 698cm -1 ; 1 H-NMR (400MHz, CDCl 3 ): δ8.02(dd, J=1.6Hz, J=8.0Hz, 1H), 7.75-7.72(m, 2H), 7.59-7.55(m, 1H), 7.48-7.38(m, 6H), 7.36- 7.30(m, 3H), 7.25-7.21(m, 1H), 5.85(d, J=4.8Hz, 1H), 4.72(d, J=4.8Hz, 1H)ppm; 3 C-NMR (100MHz, CDCl 3 ): δ161.4, 155.6, 152.7, 146.8, 143.5, 132.5, 131.9, 129.2, 128.6, 128.6, 128....
Embodiment 2
[0019] 4-Hydroxycoumarin (10 mmol) and 3-phenyl-1-(3-methoxyphenyl)-2-propyn-1-ol (15 mmol) were dissolved in nitromethane, Molecular iodine (0.8 mmol) catalyzes the reaction at a temperature of 50°C for 1 hour, then drips 0.4 milliliters of sulfuric acid, stirs overnight at 100°C, adds 20 milliliters of saturated sodium sulfite, and extracts with 20×2 milliliters of ethyl acetate at the same time. Dry over sodium sulfate, concentrate under reduced pressure, and then purify by column chromatography to obtain 3,4-(2-phenyl-4-m-methoxyphenylpyrano)coumarin with a yield of 54%. The physical data of the product are:
[0020] white solid; mp: 144-146℃; IR(KBr): 1718, 1628, 1609, 1491, 1389, 1262, 1019, 766cm -1 ;
[0021] 1 H-NMR (400MHz, CDCl 3 ): δ8.00(d, J=8.0Hz, 1H), 7.73-7.71(m, 2H), 7.58-7.54(m, 1H), 7.46-7.32(m, 1H), 7.24-7.22(m, 1H ), 7.02-6.96(m, 2H), 6.77(dd, J=2.4Hz, J=8.4Hz, 1H), 5.83(d, J=4.2Hz, 1H), 4.68(d, J=4.2Hz, 1H ), 3.77(s, 1H)ppm; 3 C-NMR (100MHz, CDCl ...
Embodiment 3
[0023] 4-Hydroxycoumarin (10 mmol) and 3-phenyl-1-p-chlorophenyl-2-propyn-1-ol (12 mmol) were dissolved in nitroethane in molecular iodine ( 2.0 mmol) and react at 80°C for 1 hour, add 0.8 ml of sulfuric acid dropwise, stir overnight at 80°C, add 20 ml of saturated sodium sulfite, extract with 20×2 ml of ethyl acetate, and dry the organic phase over anhydrous sodium sulfate , concentrated under reduced pressure, and then purified by column chromatography to obtain 3,4-(2-phenyl-4-p-chlorophenylpyran)coumarin with a yield of 61%. The physical data of the product are: yellow solid; mp: 196-197°C; IR (KBr): 1724, 1629, 1611, 1493, 1388, 1113, 1015, 760cm -1 ;
[0024] 1 H-NMR (400MHz, CDCl 3 ): δ8.02-8.00(m, 1H), 7.74-7.72(m, 2H), 7.59-7.55(m, 1H), 7.48-7.32(m, 7H), 7.28-7.25(m, 2H), 5.79 (d, J=5.6Hz, 1H), 4.68 (d, J=5.6Hz, 1H) ppm; 13 C-NMR (100MHz, CDCl 3 ): δ161.4, 155.8, 152.7, 147.1, 142.0, 133.0, 132.4, 132.1, 129.8, 129.4, 128.7, 124.6, 124.2, 122.7, 116.8, 114.3, 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com