Stable kallikrein-1 injection
A kallikrein and injection technology, which is applied in the field of solutions containing protease, can solve the problems of inconvenient clinical use, short duration of maintaining the activity of the main drug, and increased production costs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Preparation of recombinant human kallikrein-1
[0068] (Take the secretion and expression of human kallikrein-1 in the methanol yeast system as an example, other expression systems, expression methods or expression genes are used, and the basic operations are similar.)
[0069] The human kallikrein-1 gene (rhK1) obtained by PCR was inserted into the commercial vector of pPICZαA (Invitrogen) to construct the secretory expression vector pPICZα-hK1. According to the basic operations of molecular biology, the transformation and screening were performed to obtain the secretory expression vector. The rhK1 / X33 engineered strain expressing human kallikrein-1.
[0070] 1. Determine the fermentation conditions of rhK1 / X33 engineering strain in 30L tank
[0071] The seed solution was connected to a fermentation tank equipped with 15L BSM medium at a 1:15 inoculum, and the cell proliferation culture phase was started: the growth temperature was 30°C, the initial rotation speed was 300rpm, ...
Embodiment 2
[0079] Preparation of recombinant human kallikrein-1 injection I
[0080] prescription:
[0081] Recombinant human kallikrein-1 (methanol yeast) 200AU (the final concentration of activity is 0.2AU / mL)
[0082] Disodium hydrogen phosphate 1.732g
[0083] Sodium dihydrogen phosphate monohydrate 1.077g
[0085] Mannitol 10g
[0086] Dextran-40 10g
[0087] Preparation process: weigh disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride, mannitol, dextran-40 according to the amount in the above prescription, add appropriate amount of water for injection to fully dissolve the auxiliary materials, and adjust the pH value with phosphoric acid or NaOH To 7.0, add appropriate amount of recombinant human kallikrein-1 to it, mix well, dilute to 1000ml with injection water, filter and sterilize, fill, stopper, and cap to obtain recombinant human kallikrein Release Enzyme-1 Injection I.
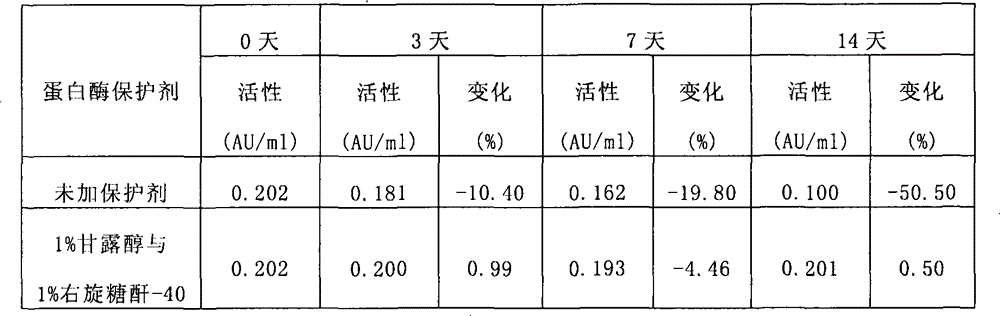
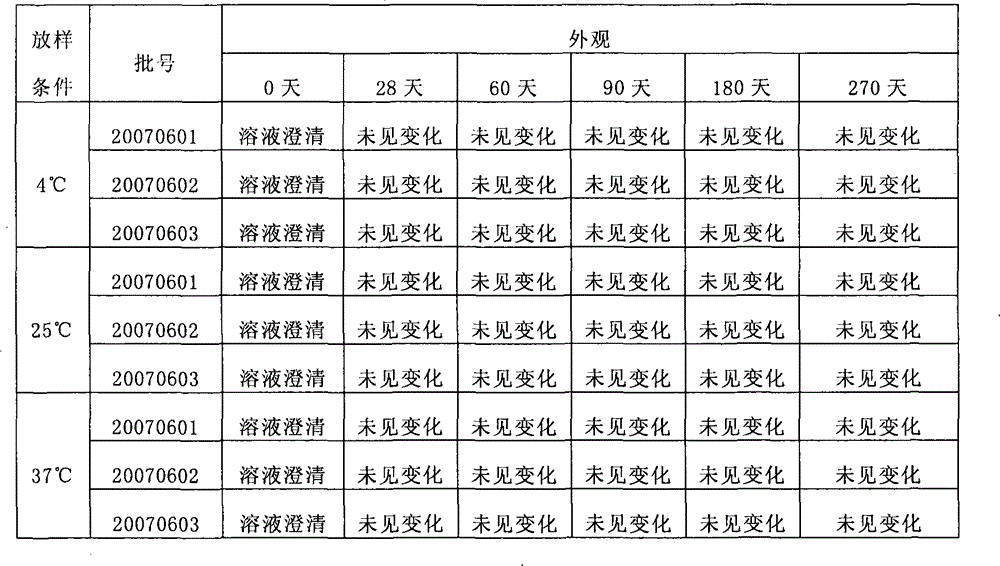
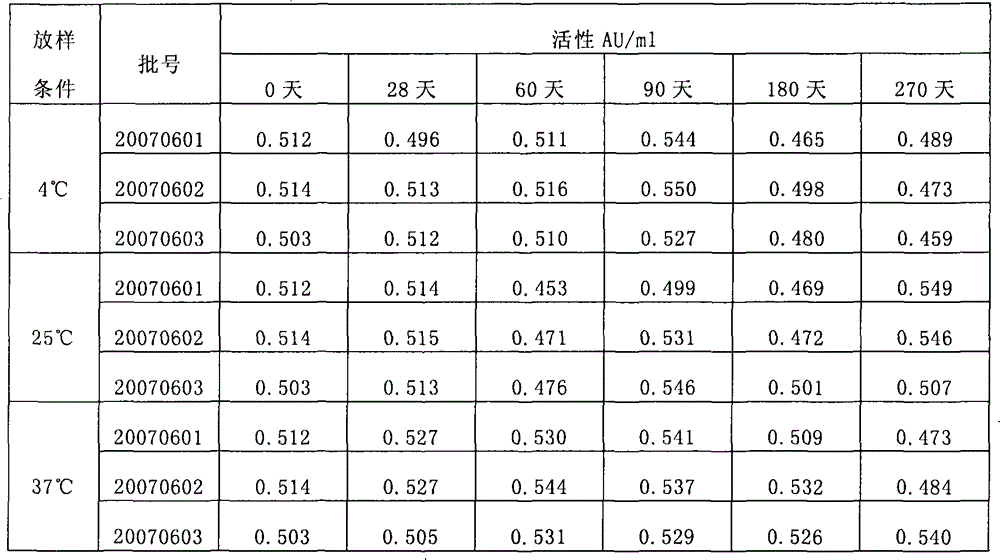
[0088] When the present invention adopts the accelerated test ...
Embodiment 3
[0099] Preparation of recombinant human kallikrein-1 injection II
[0100] prescription:
[0101] Recombinant human kallikrein-1 (CHO cells) 150AU (final concentration of activity is 0.15AU / mL)
[0102] Disodium hydrogen phosphate 1.732g
[0103] Sodium dihydrogen phosphate monohydrate 1.077g
[0104] Sodium chloride 8.775g
[0105] Hydrolyzed gelatin 10g
[0106] Preparation process: Weigh disodium hydrogen phosphate, sodium dihydrogen phosphate, sodium chloride, and hydrolyzed gelatin, add an appropriate amount of water for injection to fully dissolve the auxiliary materials, adjust the pH to 7.0 with phosphoric acid or NaOH, and then measure an appropriate amount of recombinant human gelatin Add peptidase-1, mix well, make the volume to 1000ml with injection water, filter and sterilize, fill, stopper, and cap to obtain human kallikrein-1 injection II.
[0107] The formulation used a 20mmol / L disodium hydrogen phosphate-sodium dihydrogen phosphate buffer system with pH 7.0 and 0.15 mol / ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com