Polyethylene glycol modified human interleukin-2, preparation method and application thereof
A technology of polyethylene glycol and human interleukin, which is applied in the directions of non-active ingredients medical preparations, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve problems such as loss of biological activity of drugs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] 100mg pure growth hormone, dissolved in 20ml 20mMPH6.0PB, added 400mg Y-PEG-NHS ester, reacted at 25°C for 6h, added 2M glycine to terminate the reaction. The reaction mixture was separated by S-300, detected at 250nm, and the samples were collected in sections to separate the multi-modified substance from the single-modified substance and unmodified growth hormone.
[0023] 7.5% reduced SDS-PAGE detection, the purity is greater than 95%. According to the Chinese Pharmacopoeia in 2005, the activity was reduced by 60%.
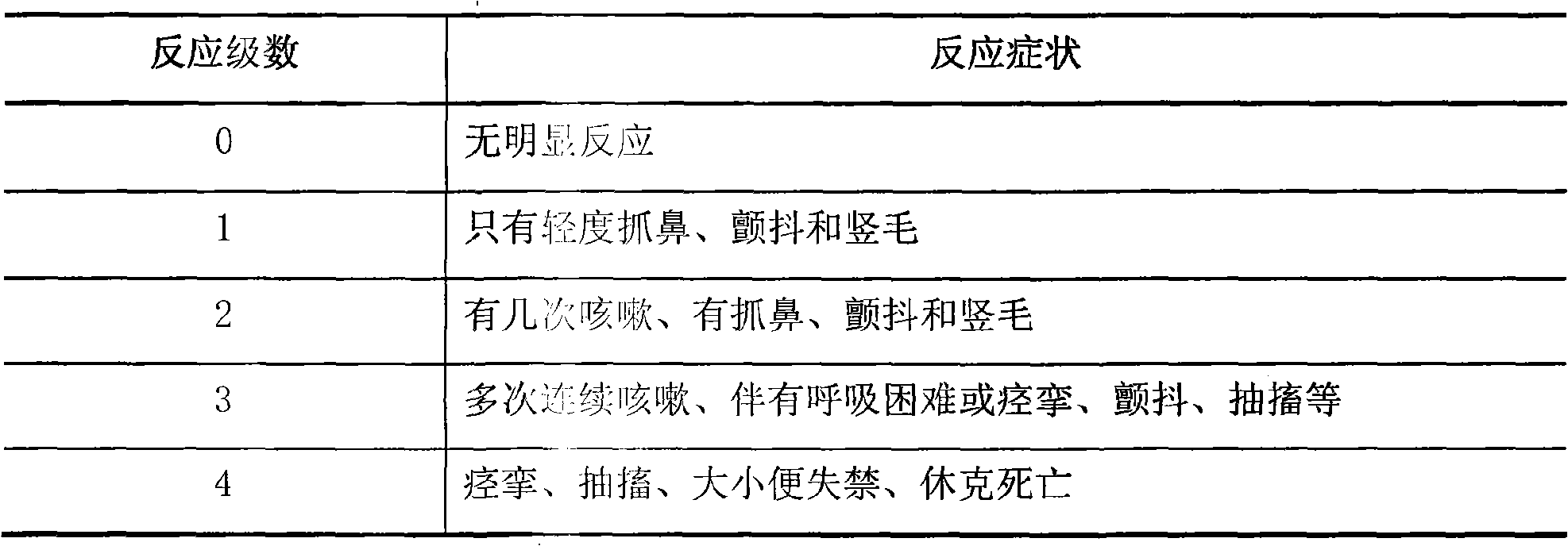
[0024] Antibody serum was collected from the immunized animals, and the antibody titer of the immune serum was determined. The results showed that the antibody titer of the modified interleukin-2 decreased, indicating that the immunogenicity of the modified interleukin-2 was reduced.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com