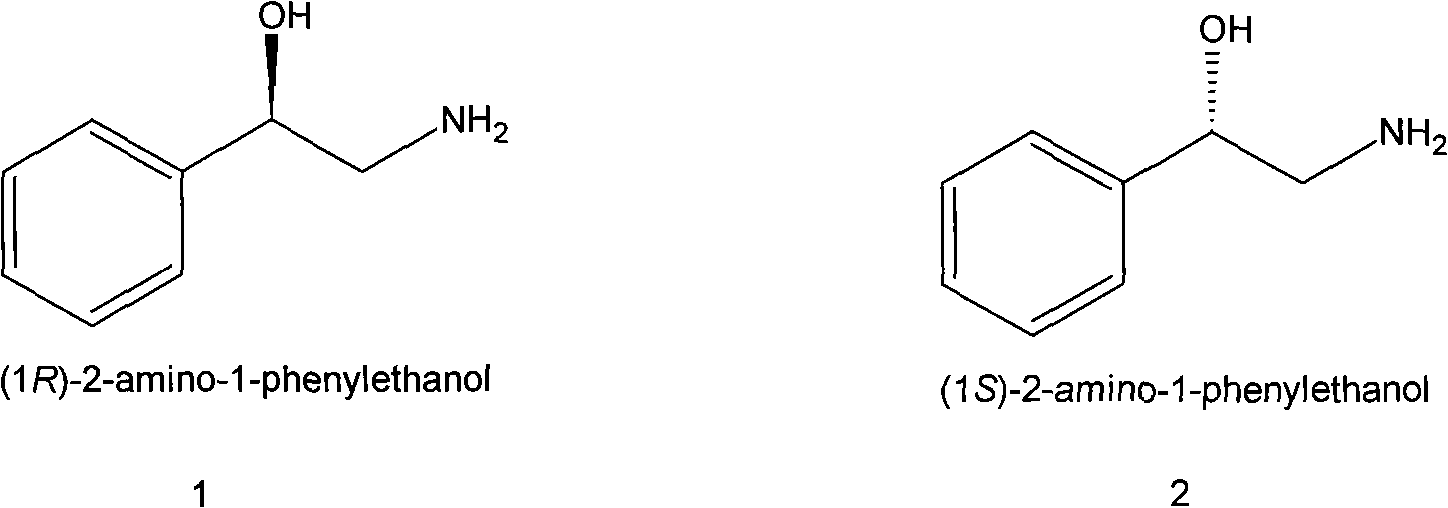
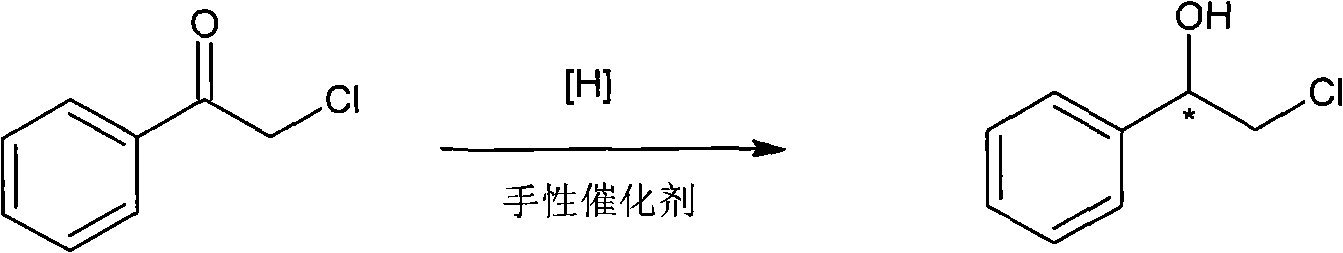
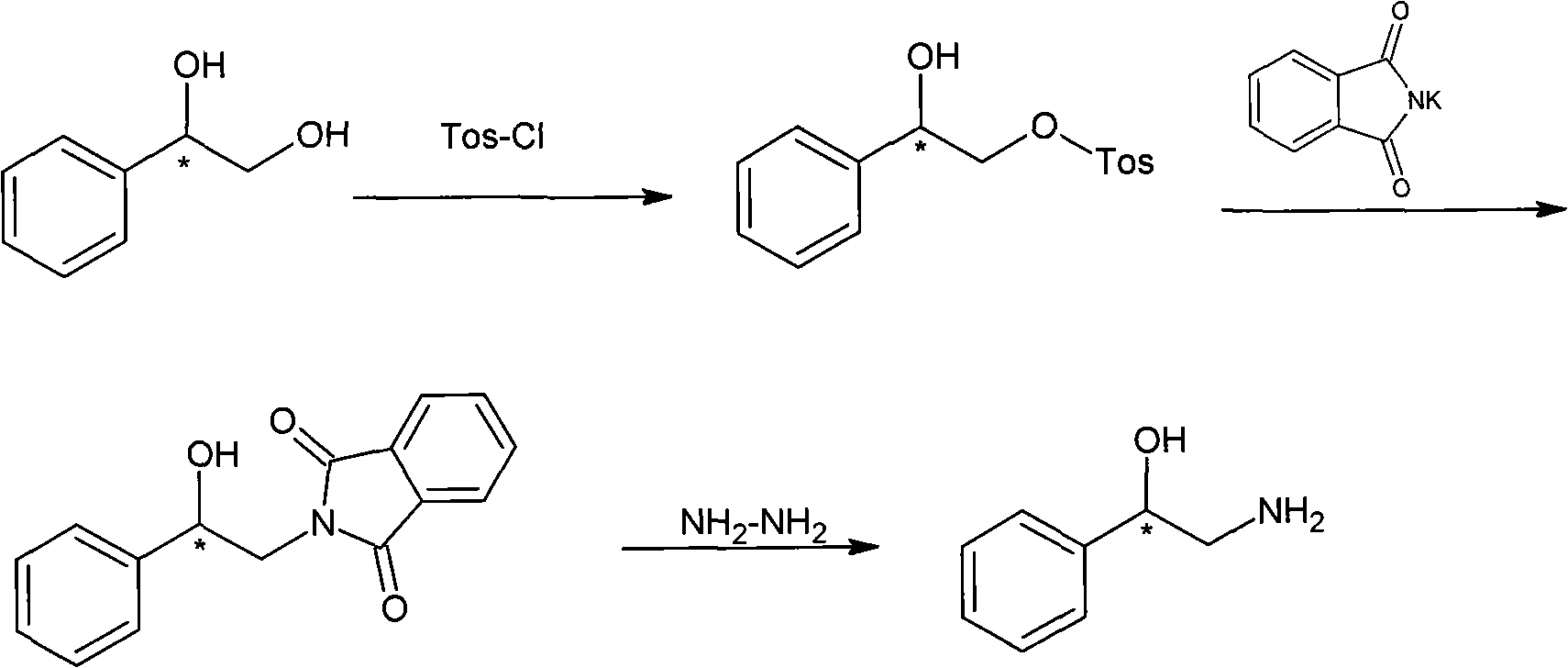
Method for preparing chiral medicinal intermediate 2-amido-1-phenylethylalcohol
A technology for an intermediate, phenethyl alcohol, is applied in the field of preparing a chiral pharmaceutical intermediate 2-amino-1-phenylethyl alcohol, which can solve the problem that specificity and specificity are difficult to apply in large-scale production, and the separation method is difficult to obtain compounds, Optical purity is difficult to achieve in one step, to achieve the effect of low cost, simple operation, high optical purity and chemical purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A method for preparing chiral pharmaceutical intermediate R-2-amino-1-phenylethanol, the method comprising the following steps:
[0028] Dissolve 15 grams (0.11mol) of commercial product R-1,2-phenylethylene glycol in 30mL of dichloromethane, then place it in an ice-water bath at about zero temperature, and quickly drop triethylamine 12.1g (0.12mol , 1.1eq), then add 57mg (0.22mmol, 0.002eq) of di-n-butyltin oxide, and finally add 21.3g (0.11mol, 1eq) of p-toluenesulfonyl chloride 25mL dichloromethane solution dropwise, and keep the temperature during the dropwise addition Between 25°C and 30°C. After the dropwise addition was completed, react overnight at room temperature. After the reaction was completed, pour into 25 mL of water, then separate the layers, wash the water layer with 15 mL×3 dichloromethane, wash the organic layer with water for 3 times, then dry over anhydrous sodium sulfate, suction filter, and spin Remove dichloromethane, and then petroleum ether / et...
Embodiment 2
[0032] A method for preparing chiral pharmaceutical intermediate S-2-amino-1-phenylethanol, the method comprising the following steps:
[0033] 5 grams (0.036mol) of S-1,2-phenylethylene glycol were dissolved in 15mL of dichloromethane, then placed in a salt ice bath and cooled to minus 15 degrees, and slowly added dropwise 6.1g (0.06mol, 1.7eq), then dropwise add 10.6g (0.055mol, 1.5eq) of p-toluenesulfonyl chloride in 15mL dichloromethane solution, keep the temperature at -10°C during the dropwise addition process, naturally heat up after the dropwise addition, and react overnight at room temperature. Rinse into 15mL water after completion, separate the liquids, wash the aqueous layer with 10mL×3 dichloromethane, collect the organic layer, wash 3 times with water, dry over anhydrous sodium sulfate, filter with suction, spin off the dichloromethane, wash with petroleum ether / acetic acid Ethyl ester was recrystallized to obtain (S)-2-p-toluenesulfonic acid-1-phenyl-1,2-ethaned...
Embodiment 3
[0037] A method for enlarging the preparation of chiral pharmaceutical intermediate R-2-amino-1-phenylethanol, the method comprising the following steps:
[0038] Take 1.5kg (10.87mol) of R-1,2-phenylethylene glycol and dissolve it in 3000mL of dichloromethane, place it in an ice-water bath, quickly add 1.21kg (11.96mol, 1.1eq) of triethylamine dropwise, and then add Di-n-butyltin oxide 5.7g (22mmol, 0.002eq), add dropwise 2500mL methylene chloride solution of p-toluenesulfonyl chloride 2.13kg (11.20mol, 1.03eq), keep the temperature between 25°C and 30°C during the dropwise addition The dropwise addition is completed in about 3-4 hours. After the dropwise addition is completed, the room temperature is reacted overnight. Wash, dry over anhydrous sodium sulfate and decolorize with activated carbon, filter with suction, spin off dichloromethane, recrystallize from petroleum ether / ethyl acetate to obtain (R)-2-p-toluenesulfonic acid-1-phenyl-1,2- Ethylene glycol 1.7kg, yield 53%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com