Magnetic fluorescent composite nanoparticle, as well as preparation and use thereof
A composite nanoparticle and nanoparticle technology, which is applied in the field of biomedicine and nanomaterials, can solve the problems of difficult control of composite particle size and easy aggregation of magnetic particles, etc., and achieve the reduction of cell magnetic resonance signal, long-lasting fluorescence intensity, and high fluorescence intensity. strong effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] (1) Hydrophobic Fe 3 o 4 Preparation of nanoparticles: Under nitrogen protection, quickly mix the mixture of 0.5mmol iron acetylacetonate, 2.5mmol 1,2-hexadecanediol, 1.5mmol oleic acid, 1.5mmol oleylamine and 5ml benzyl ether, at 200°C After keeping warm for 2 hours, continue to heat to 300°C, reflux for 1 hour, and cool to room temperature. The product is precipitated with absolute ethanol, centrifuged at 10000rpm / min for 5 minutes, and the precipitate is dissolved in a solution containing 0.013ml oleic acid and 0.013ml oleylamine In 10ml of n-hexane, centrifuge at 6000rpm / min for 10 minutes, and disperse the precipitate in 2ml of n-hexane to obtain 6nm primary hydrophobic Fe 3 o 4 Nanoparticles; Next, the hydrophobic Fe of 10nm is prepared according to the following steps 3 o 4 Nanoparticles: A, containing 20mg6nm primary hydrophobic Fe 3 o 4 Add 0.5mmol iron acetylacetonate, 2.5mmol 1,2-hexadecane diol, 0.5mmol oleic acid, 0.5mmol oleylamine and 5ml benzyl eth...
Embodiment 2
[0040] (1) Hydrophobic Fe 3 o 4 Preparation of nanoparticles: prepare 6nm hydrophobic Fe in the same way as in Example 1 step (1) 3 o 4 particle.
[0041] (2) Hydrophilic Fe 3 o 4 The preparation of nanoparticle: prepare 6nm hydrophilic Fe by the same method of embodiment 1 step (2) 3 o 4 particle.
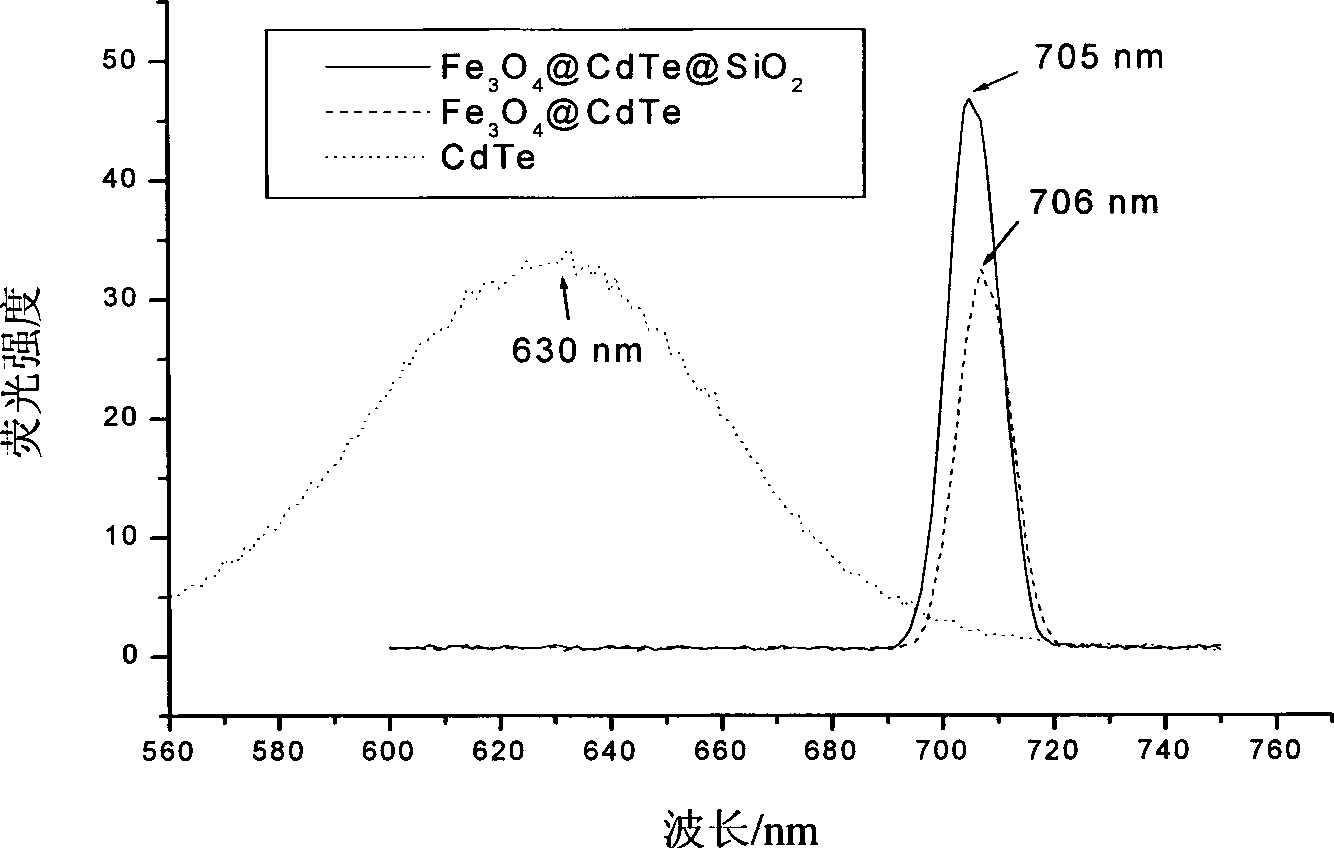
[0042] (3) Preparation of CdTe fluorescent quantum dots: prepared by the same method as in step (3) of Example 1, the difference being that the reflux time was 10 minutes, and the aqueous dispersion of CdTe fluorescent quantum dots with a fluorescence emission peak of 521nm was obtained.
[0043] (4) Fe 3 o 4 / Preparation of CdTe nanoparticles: take 6nm hydrophilic Fe 3 o 4 Nanoparticles and the quantum dot of fluorescence emission wavelength are 521nm, prepare Fe by the same method of embodiment 1 step (4) 3 o 4 / CdTe nanoparticles.
[0044] (5) Magnetic fluorescent composite nanoparticles Fe 3 o 4 / CdTe / SiO 2 Preparation: Prepared by the same method as in Examp...
Embodiment 3
[0046] (1) Hydrophobic Fe 3 o 4 Preparation of nanoparticles: prepare 8nm hydrophobic Fe in the same way as in Example 1 step (1) 3 o 4 particle.
[0047] (2) Hydrophilic Fe 3 o 4 The preparation of nanoparticle: prepare 8nm hydrophilic Fe by the same method of embodiment 1 step (2) 3 o 4 particle.
[0048] (3) Preparation of CdTe fluorescent quantum dots: prepared by the same method as in Example 1 step (3), the difference being that the reflux time was 1 hour to obtain a CdTe fluorescent quantum dot aqueous dispersion with a fluorescence emission peak of 560 nm.
[0049] (4) Fe 3 o 4 / Preparation of CdTe nanoparticles: take 8nm hydrophilic Fe 3 o 4 Nanoparticles and CdTe fluorescent quantum dots with a fluorescence emission wavelength of 560nm were prepared in the same manner as in step (4) of Example 1 to prepare Fe3O4 / CdTe nanoparticles.
[0050] (5) Magnetic fluorescent composite nanoparticles Fe 3 o 4 / CdTe / SiO 2 Preparation of: Fe 3 o 4 Add 3 ml of eth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com