Recombinant or transgenic factor VII composition, each factor VII molecule having two N-glycosylation sites with defined glycan units
A technology of glycosylation sites and compounds, which can be used in medical preparations containing active ingredients, drug combinations, genetic engineering, etc., and can solve problems such as difficult treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0175] Example 1: Production of human FVII protein in the milk of transgenic female rabbits
[0176] First, the plasmid p1 was introduced into the Bam H1-HindIII (6.3Kb fragment) sequence of the WAP gene (described in the document Devinoy et al, Nucleic Acids Research, vol. 16, no. 16, 25 August 1988, p. 8180). The multiple linker of the p-poly III-I vector between the sequence Bam H1 and Hind III (described in the document Lathe et al, Gene (1987) 57, 193-201) was prepared.
[0177] During this cloning process, the site Bam H1 was suppressed and replaced by the site Cla I in the vector p1. Therefore, the vector p1 is able to accept foreign genes, and it is placed to rely on the 6.3Kb WAP promoter. The introduced foreign gene can be introduced, for example, to the Sal I site of the polylinker. The insert containing the promoter and the entire foreign gene can be separated from the plasmid after cutting the two Not 1 sites, which are located at the end of the polylinker of the p-po...
Embodiment 2
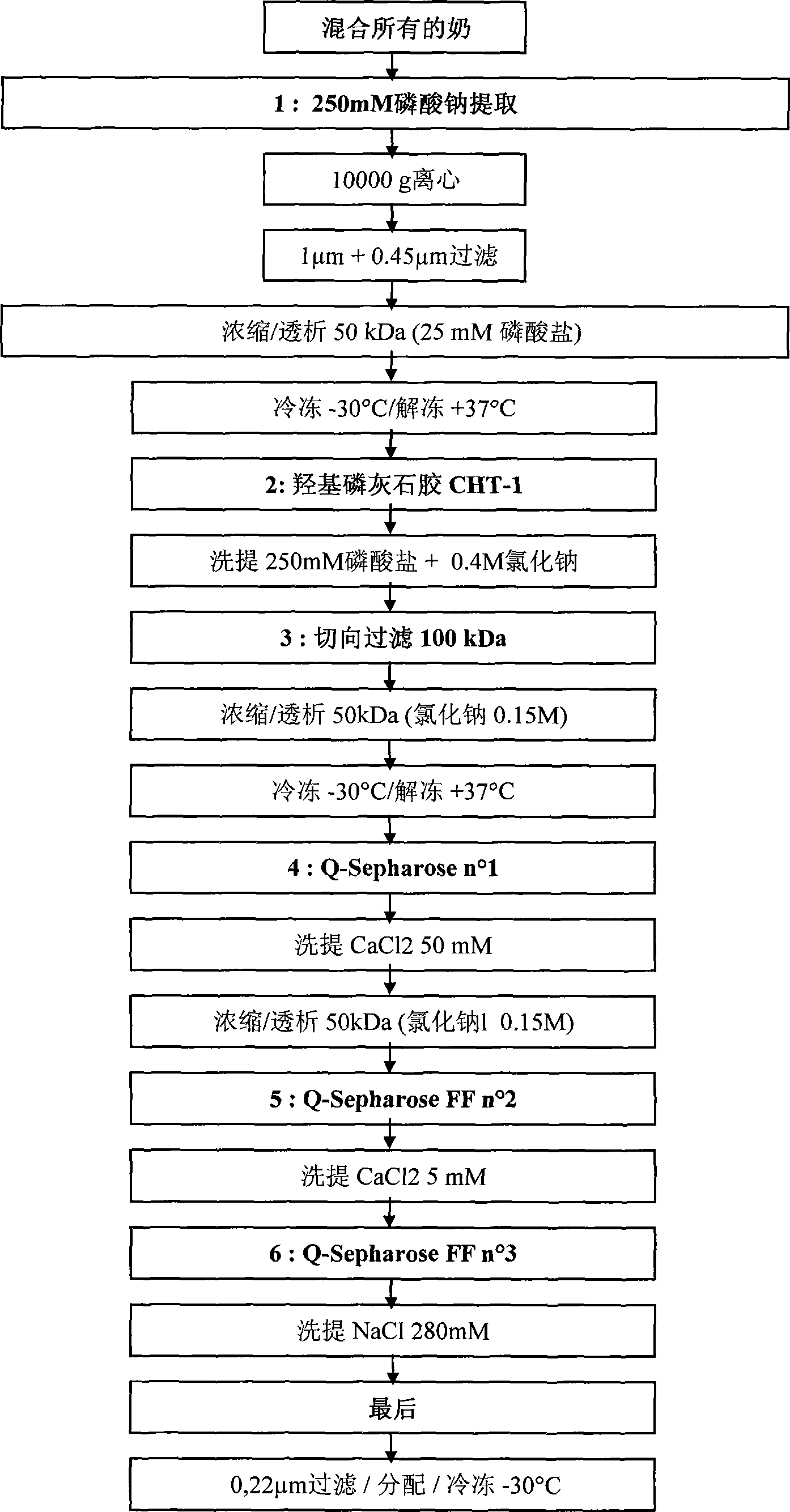
[0183] Example 2: Extraction and purification of the obtained FVII
[0184] a) Extraction of FVII
[0185] Take 500ml of whole raw milk and dilute with 9 times volume of 0.25M, pH8.2 sodium phosphate buffer. After stirring for 30 minutes at room temperature, the FVII-rich aqueous phase was centrifuged at 10,000 g at 15°C for 1 hour (centrifuge Sorvall Evolution RC-6700rpm-rotor SLC-6000). 6 cans of approximately 835ml are required.
[0186] After centrifugation, there are three phases: a fat phase on the surface (milk fat), a non-fat aqueous clear FVII-rich phase (main phase), and a white granular solid phase (insoluble casein and calcium compound precipitation).
[0187] The non-fat aqueous phase containing FVII is collected by a persistent pump until the lipid phase. The lipid phase is recovered separately. The solid phase (precipitation) is removed.
[0188] However, the non-fat aqueous phase still contains a very small amount of lipids, which is filtered on a series of filters...
Embodiment 3
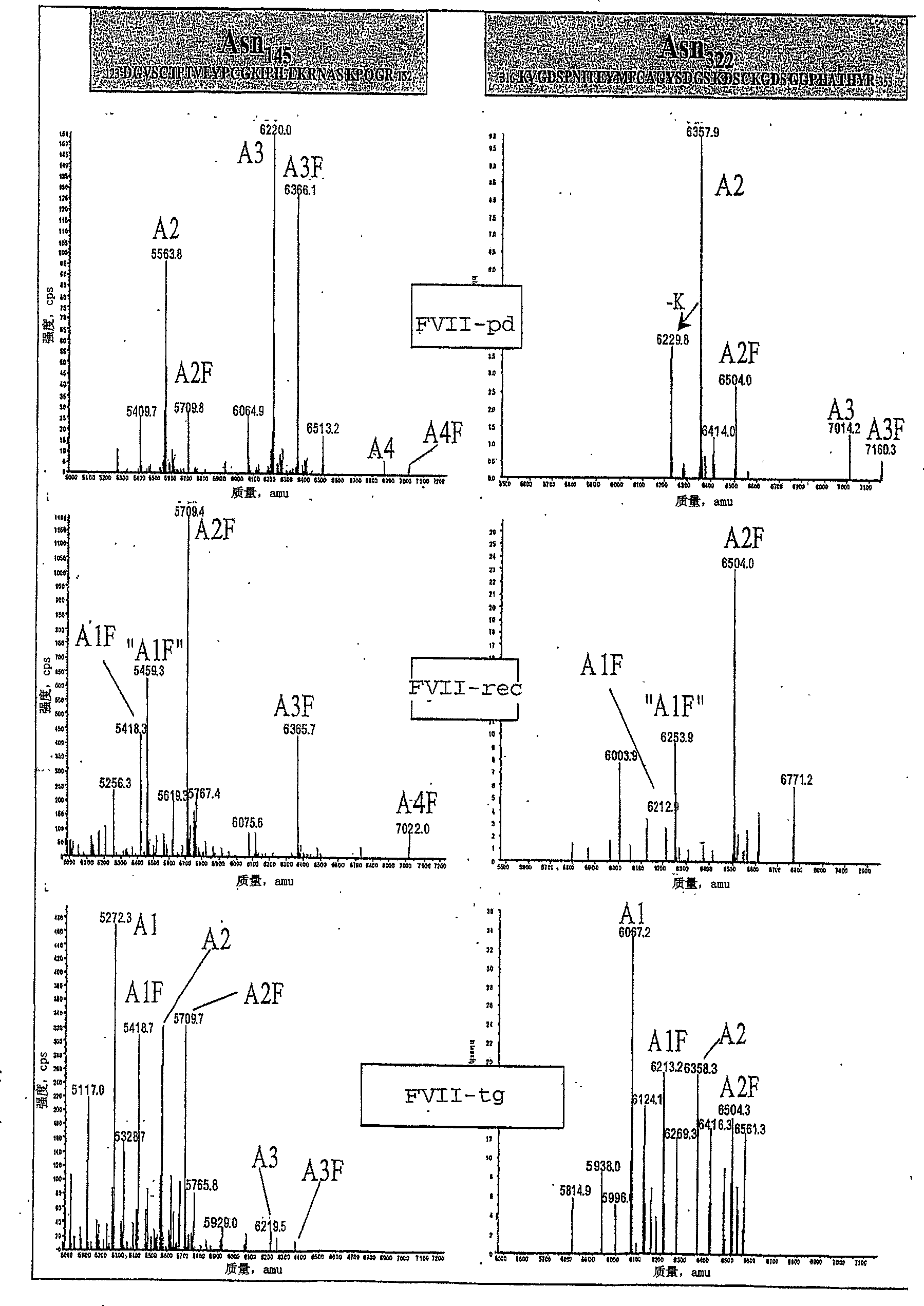
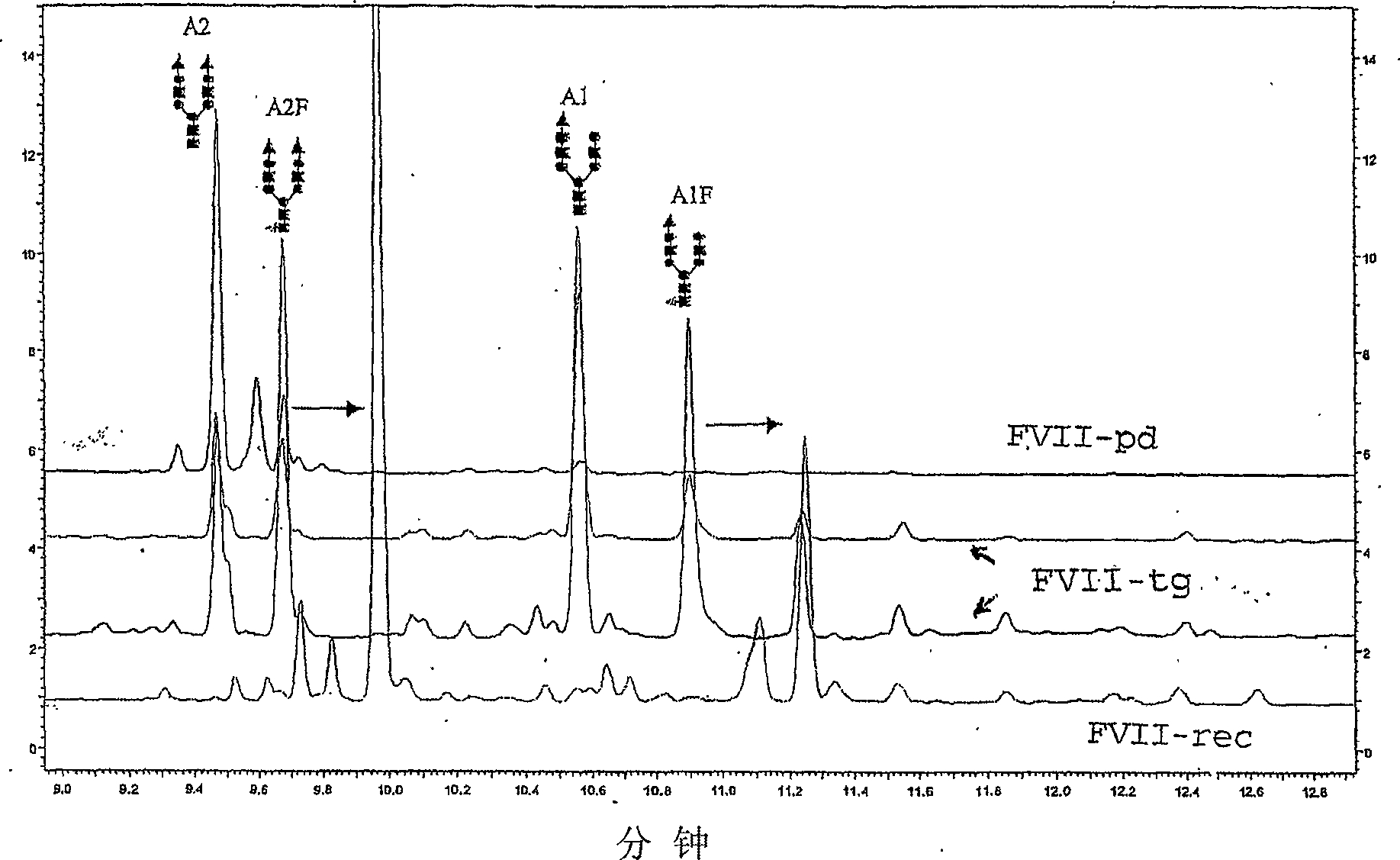
[0233] Example 3: Study of basic sequence
[0234] 1. Peptide map
[0235] After trypsin and Asp-N (data not shown) decomposed samples FVII-pd, FVII-rec and FVII-tg, LC-MS and UV detection chromatograms were obtained.
[0236] Analysis of the peptide maps obtained after the degradation of trypsin showed that they were similar within a retention time of less than 65 minutes. Later, the species corresponding to the peptide heavy chain fragment was found in FVII-rec and FVII-tg but not in FVII-pd. These high-quality peptides (4500 Da to 8000 Da) correspond to incomplete cleavage. These peptides have been found in other batches of FVII-pd, therefore, their presence is not associated with different trypsin sensitivity.
[0237] The peptide maps obtained after Asp-N degradation are very similar between different FVIIs, and this is true along the entire retention time series. The observed difference in intensity is only due to the change in the amount of injection and has nothing to do...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com