Wholly aromatic side chain type sulfonated diamine and preparation method thereof
A sulfonated diamine and side chain technology, which is applied in the field of fully aromatic side chain sulfonated diamine and its preparation, can solve the problem that it is difficult to meet the actual use requirements, the resistance to free radical oxidation and thermal stability is deteriorated, and the research results Issues such as public reports, to achieve the effects of excellent mechanical properties, excellent film-forming properties, and wide application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of 2,2'-bis(4-sulfophenoxy)biphenyldiamine
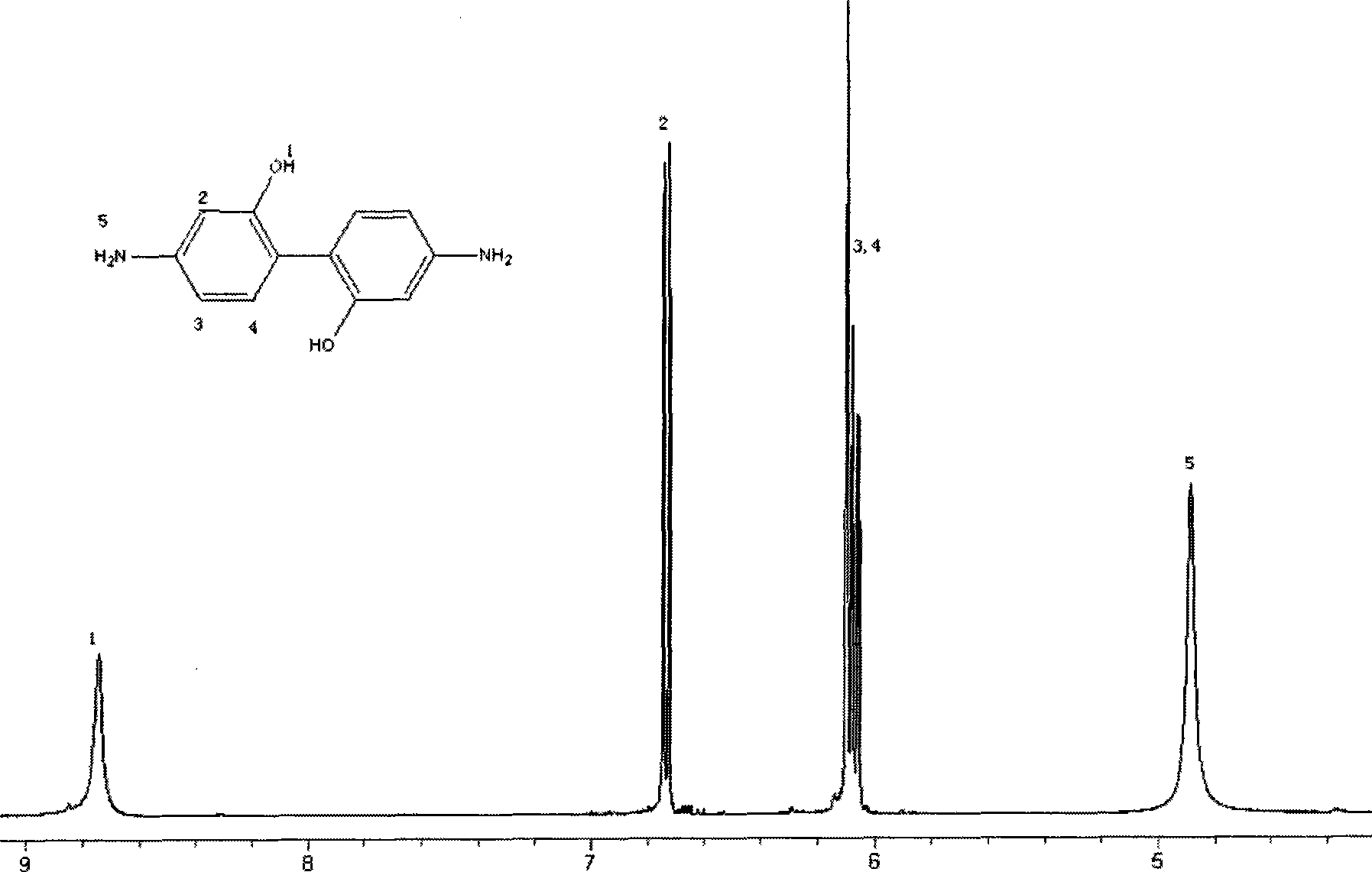
[0031] Under the conditions of nitrogen protection and magnetic stirring, add 6.95g (0.05mol) 3-nitrophenol, 13g (0.2mol) zinc powder, 50ml ethanol, 25ml (12mol / L) sodium hydroxide solution in the dry reaction flask, React for 10 hours, cool, add 45ml of concentrated hydrochloric acid, stir for 10 hours, add 25ml (12mol / L) sodium hydroxide solution, filter with suction, add 30ml of glacial acetic acid to the filtrate, collect the precipitate, and wash with deionized water until neutral , dried to obtain 3.8g of product, the yield was 70%. The resulting products were characterized structurally, including 1 H NMR spectrum, see figure 1 , proving that the synthesized product is 2,2'-dihydroxybenzidinediamine.
[0032] The specific characterization results are as follows:
[0033] figure 1 is 2,2'-dihydroxybenzidinediamine 1 HNMR with DMSO-d 6 It is the solvent, and the attribution of each peak is indicated in ...
Embodiment 2
[0039] Preparation of 2,2'-bis[4-(4-sulfonylsulfone)phenoxy]biphenyldiamine
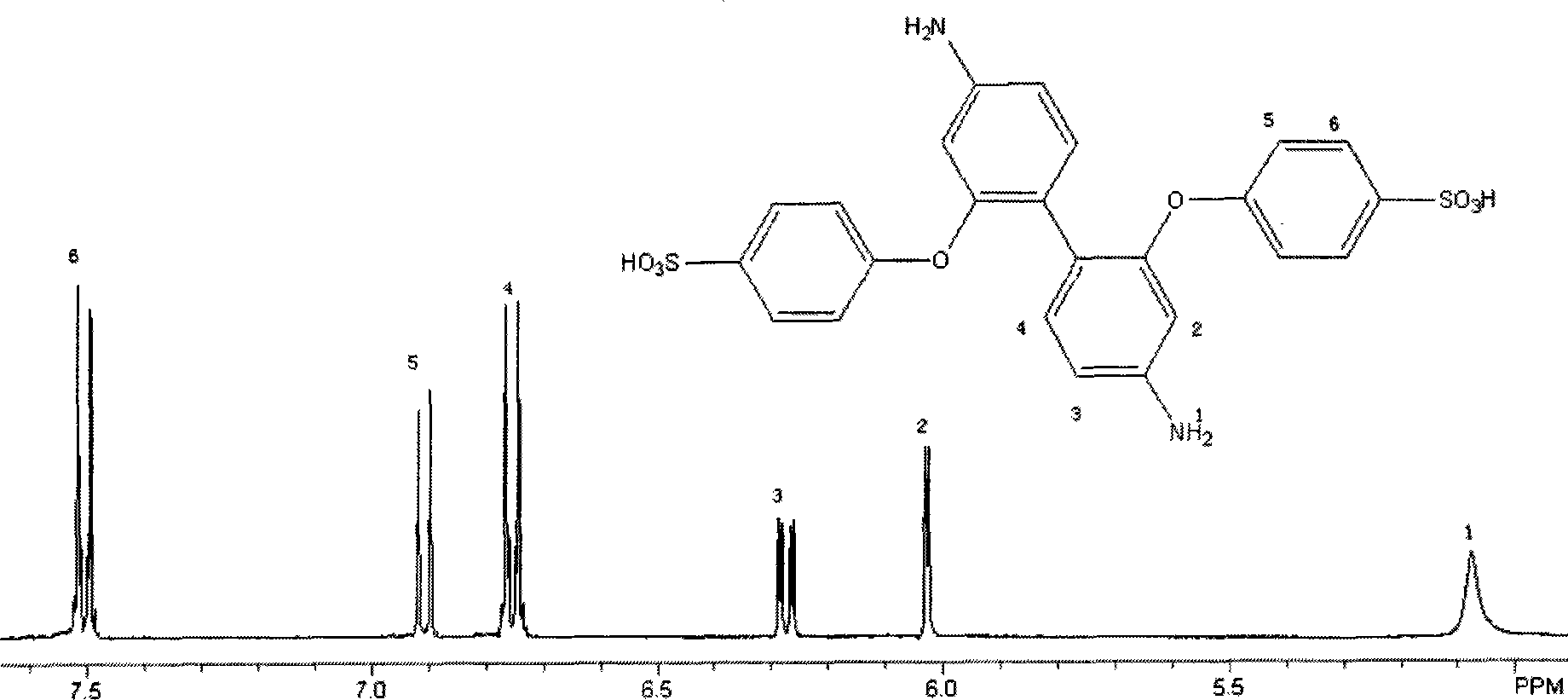
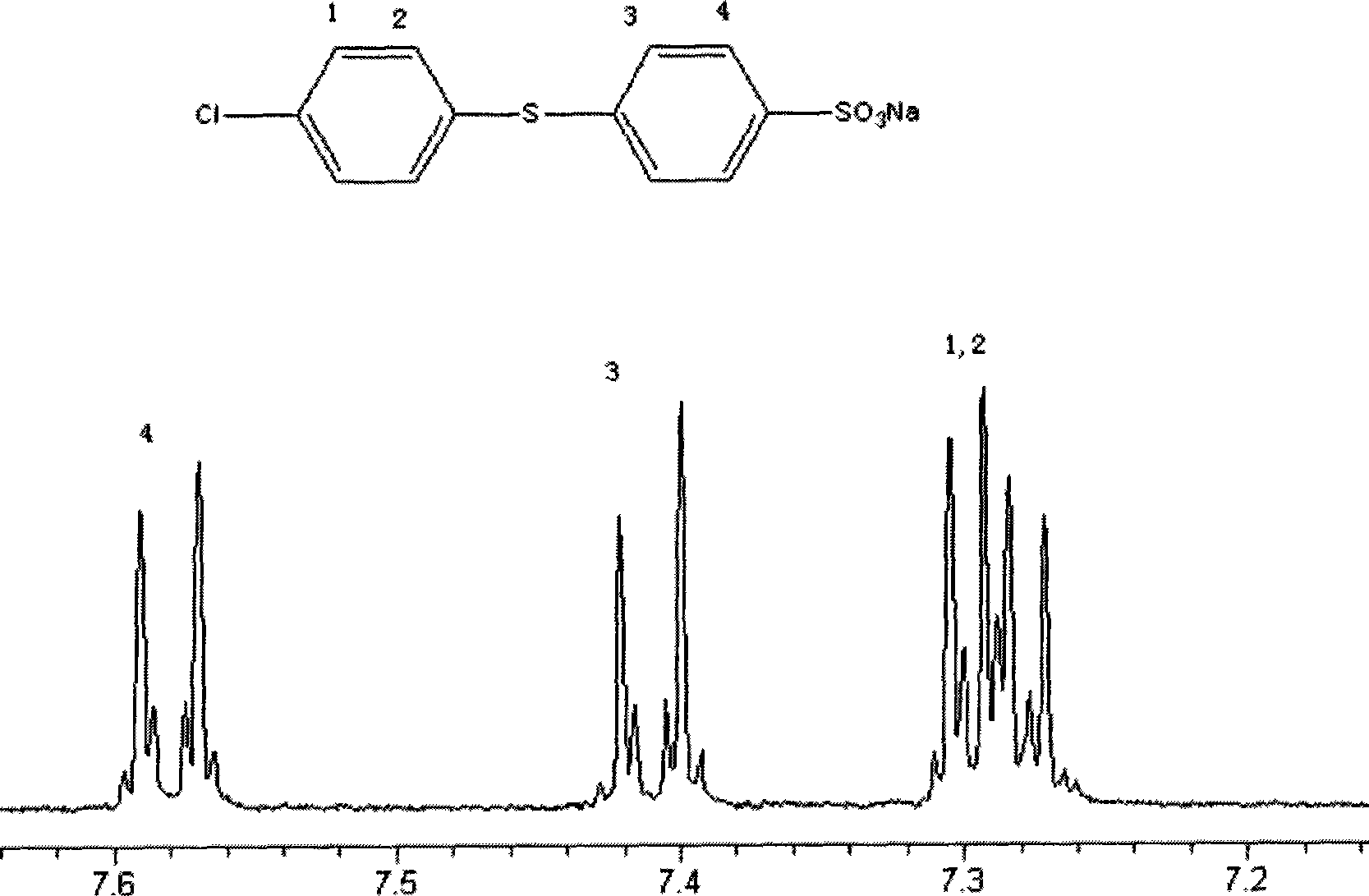
[0040]Under the conditions of nitrogen protection and magnetic stirring, 1.98g (0.01mol) sodium p-fluorobenzenesulfonate, 1.445g (0.01mol) p-chlorothiophenol, 4g anhydrous potassium carbonate, 10mL dimethyl methoxide were added to the dry reaction flask. sulfone and 10mL toluene, heat the reaction system to 150°C, distill the toluene and water, and react at this temperature for 20 hours. After the reaction, cool the reaction system to room temperature, then pour it into 300mL acetone, filter with suction, and The obtained solid was dissolved in 100 mL of hot water, suction filtered, the filtrate crystallized, the precipitate was collected, washed with deionized water until neutral, and dried to obtain 2.3 g of the target product with a yield of 70%. The resulting products were characterized structurally, including 1 H NMR see, image 3 , proving that the synthesized product is 4-chlorodiphenylsulfi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com