Skin cosmetic
A technology for cosmetic materials and compounds, applied in cosmetics, skin diseases, cosmetic preparations, etc., can solve the problems of insufficient solubility of bisphenol compounds, limited use range, poor permeability, etc., and achieves excellent melanin production inhibition effect, easy application, No skin irritation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
[0035] Anhydrous tetrahydrofuran was added to 5.0 g of 2,2'-dihydroxy-5,5'-di-n-propylbiphenyl (hereinafter referred to as "propyl bisphenol") used as a raw material for cosmetics, and stirred under ice cooling. Then, 1.0 ml of pyridine and 6.0 g of acetyl chloride were sequentially added, and after 4 hours, post-treatment was carried out according to the usual method to obtain 5.2 g of crude product.
[0036] The obtained product was separated by silica gel column chromatography [mobile phase: ethyl acetate / n-hexane (1:5)] using silica gel 60N (100-210μm) manufactured by Kanto Chemical Co., Ltd., and 3.0 g of monochromate were obtained as light yellow oils. Acetate and 1.9g diacetyl.
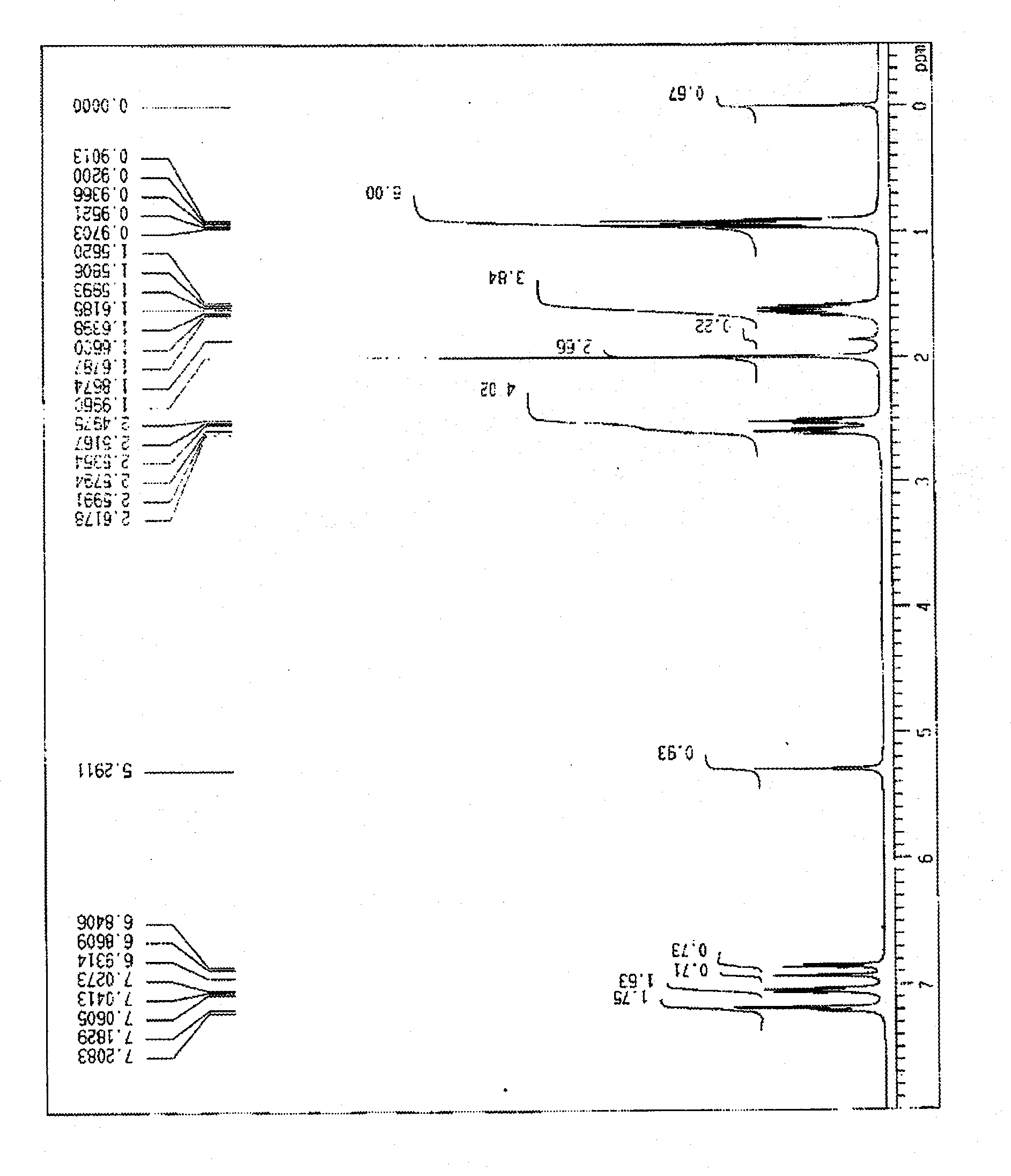
[0037] use 1 H-NMR (400MHz, CDCl 3 ) Confirm the obtained monoacetyl and diacetyl of propyl bisphenol ( figure 1 ,figure 2). The obtained NMR signal is shown below.
[0038] (Monoacetyl)
[0039]
[0040] [δ(ppm); 0.94(m, 6H), 1.63(m, 4H), 1.99(s, 3H), 2.51(t, J=7.5Hz, 2H), 2.59(t, J=7.5Hz, 2H) ...
preparation example 2
[0045] According to Preparation Example 1, anhydrous tetrahydrofuran was added to 2.2 g of propyl bisphenol and stirred under ice cooling. Then 0.5ml of pyridine and about 3.5ml of propionyl chloride were added successively, and after 4 hours, the post-treatment was carried out according to the usual method to obtain 2.2g of crude product.
[0046] The crude product was separated by silica gel column chromatography [mobile phase: ethyl acetate / n-hexane (1:4)] using silica gel 60N (100-210 μm) manufactured by Kanto Chemical Co., Ltd. to obtain 1.47 g of dipropylene as a colorless oily substance Acylate.
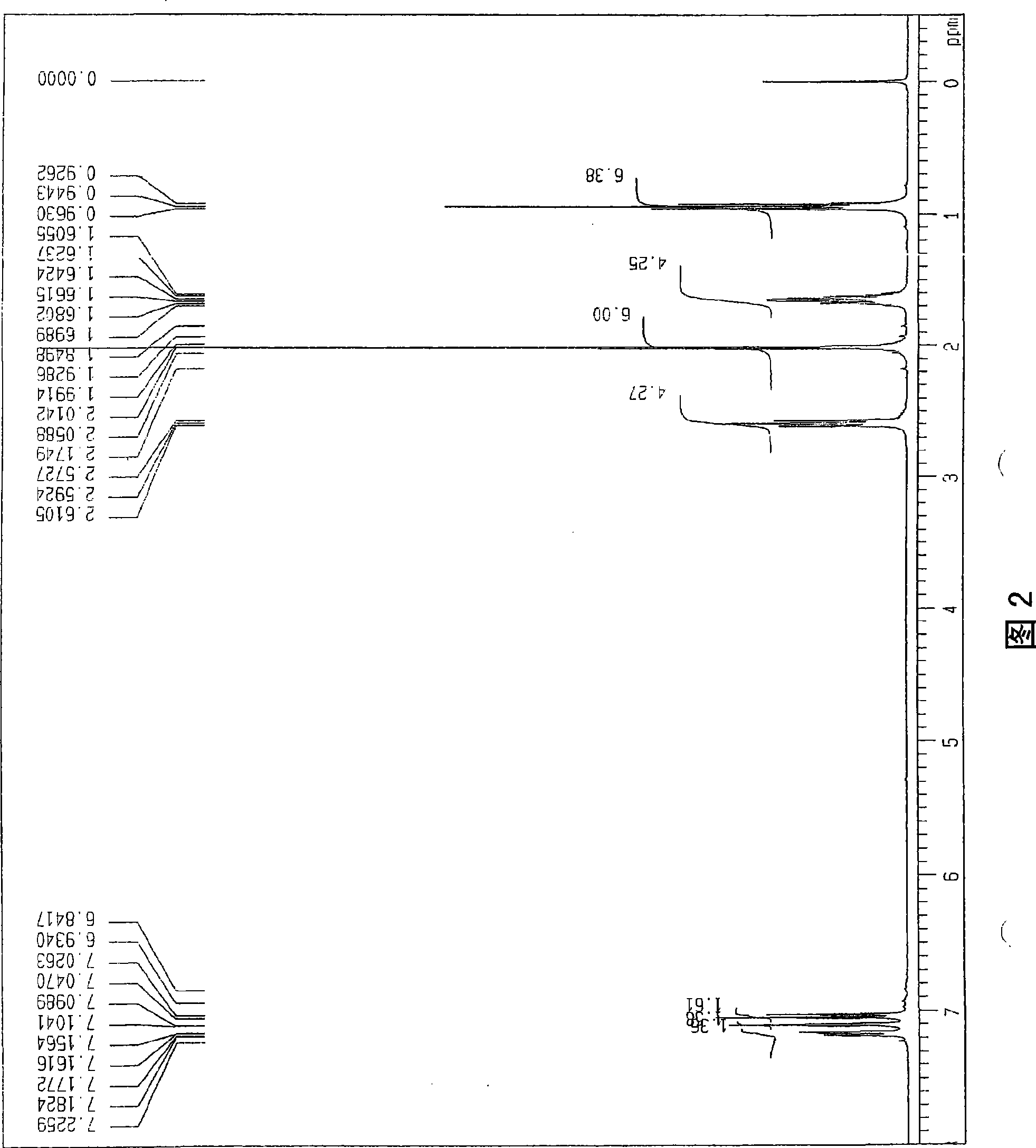
[0047] use 1 H-NMR (400MHz, CDCl 3 ) The obtained dipropionyl product of propyl bisphenol was confirmed (Figure 3). The obtained NMR signal is shown below.
[0048] (Dipropionyl)
[0049]
[0050] [δ(ppm); 0.93(t, J=7.2Hz, 6H), 0.98(t, J=7.6Hz, 6H), 1.64(q, J=7.2Hz, 4H), 2.28(q, J=7.2Hz , 4H), 2.59 (t, J = 7.2 Hz, 4H), 7.02 (d, J = 8.4 Hz, 2H), 7.08 (s, 2H), 7.16 (dd, J = 2.0, 8...
preparation example 3
[0052] According to Preparation Example 1, anhydrous tetrahydrofuran was added to 1.0 g of propyl bisphenol, and the mixture was stirred under ice cooling. Then 0.5ml of pyridine and about 2.5ml of octanoyl chloride were added successively, and after 4 hours, after treatment according to the usual method, 1.5g of crude product was obtained.
[0053] The crude product was separated by silica gel column chromatography [mobile phase: ethyl acetate / n-hexane (1:10)] using silica gel 60N (100-210μm) manufactured by Kanto Chemical Co., Ltd. to obtain 1.25 g of monooctane as a pale yellow oily substance Acylate.
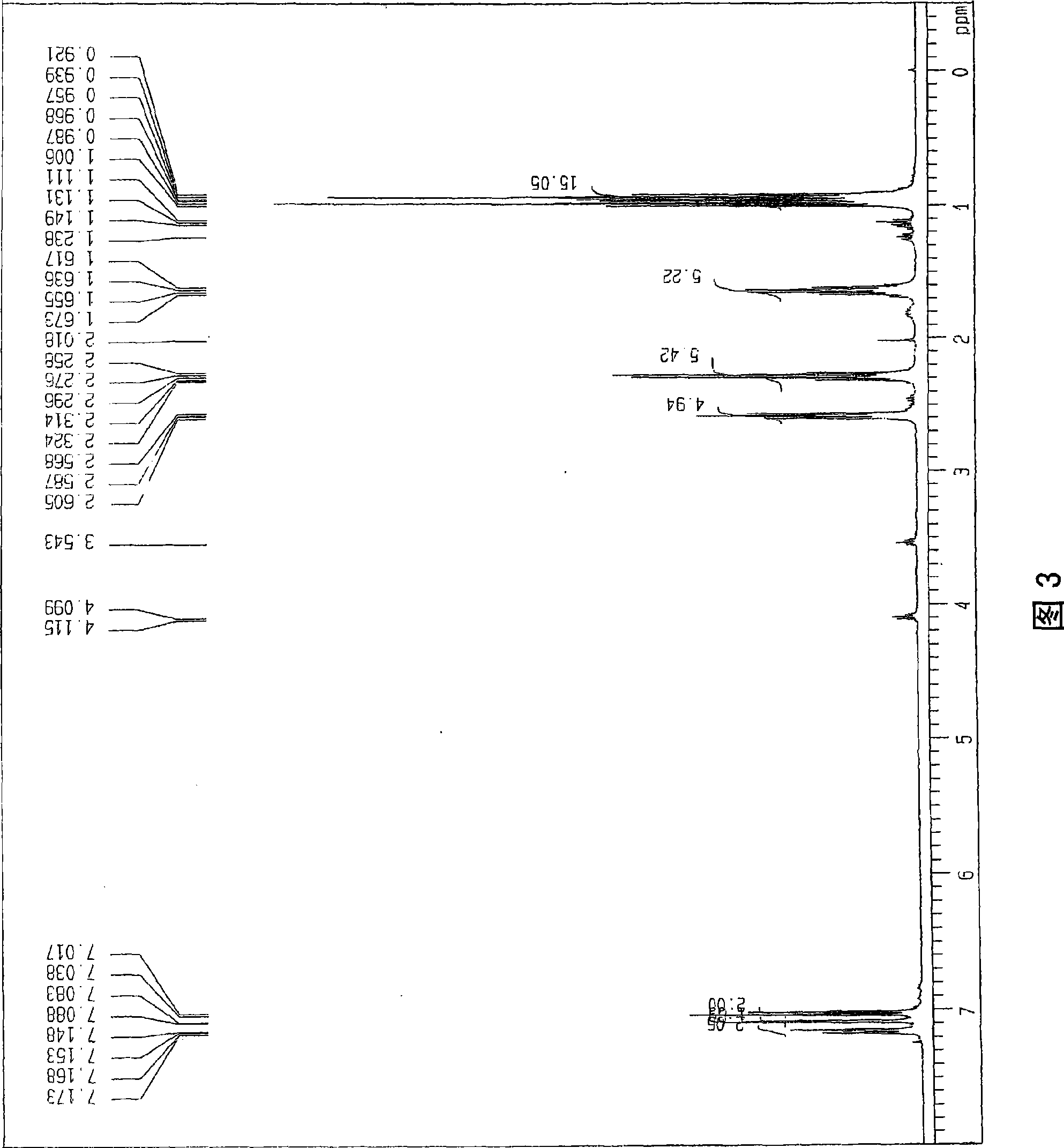
[0054] use 1 H-NMR (400MHz, CDCl 3 ) The monooctanoyl compound of the obtained propyl bisphenol was confirmed (Figure 4). The obtained NMR signal is shown below.
[0055] (Monoctanoyl)
[0056]
[0057] [δ(ppm); 0.85-0.97(m, 9H), 1.14-1.26(m, 8H), 1.41(q, J=7.2Hz, 2H), 1.58-1.69(m, 4H), 2.27(t, J =7.2Hz, 2H), 2.49-2.63 (m, 4H), 6.87-6.91 (m, 2H), 7.02-7.07 (m, 2H), 7.23-7.25 (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com