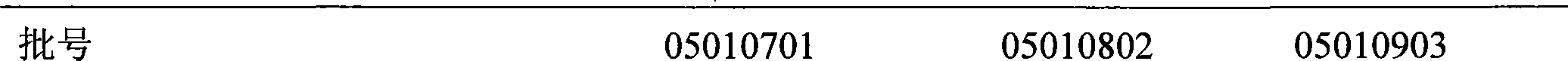Medicine composition capable of eliminating the mass and relieving swelling, absorbing clots and alleviating pain, preparation method and quality control method thereof
A quality control method, a technology for removing blood stasis and relieving pain, which is applied to drug combinations, medical preparations containing active ingredients, and pharmaceutical formulas, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0038] Experimental example 1 Process experimental research
[0039] 1. Crushing fineness test
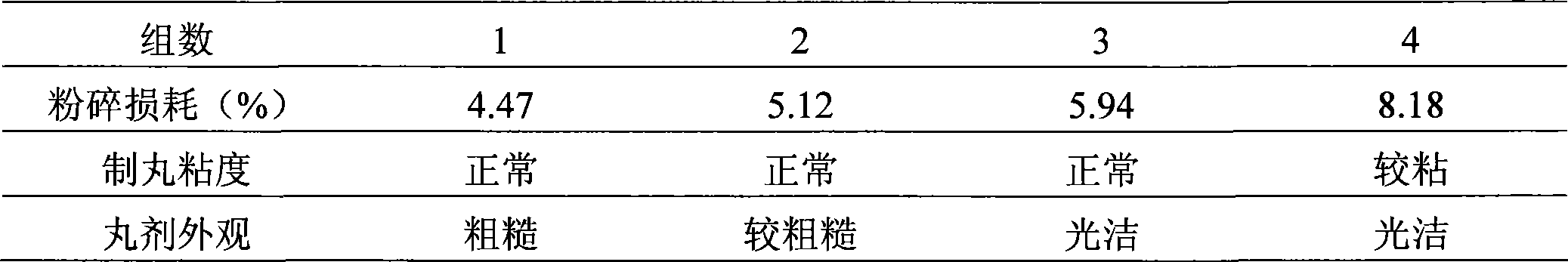
[0040] Prepare the medicinal materials according to the prescription, each containing 10g musk, 90g cochinchinensis (without the shell and oil), 90g sesame root, 90g sweetgum, frankincense (preparation) 40g, myrrh (preparation) 40g, Wulingzhi (vinegar) Stir-fried) 90g, angelica (wine stir-fried) 40g, earthworm 90g, and fragrant ink 8g, respectively, are divided into four groups for experiments: the grinding fineness is: 50 mesh, 65 mesh, 80 mesh, 100 mesh, respectively, according to the pellet viscosity, The appearance of pellets and crushing loss are indicators to determine the fineness of crushing. The results are shown in Table 1:
[0041] Table 1: Crushing test results
[0042]
[0043] The above results show that when the crushing fineness is 80 meshes, the indicators are better, so 80 meshes are used in production.
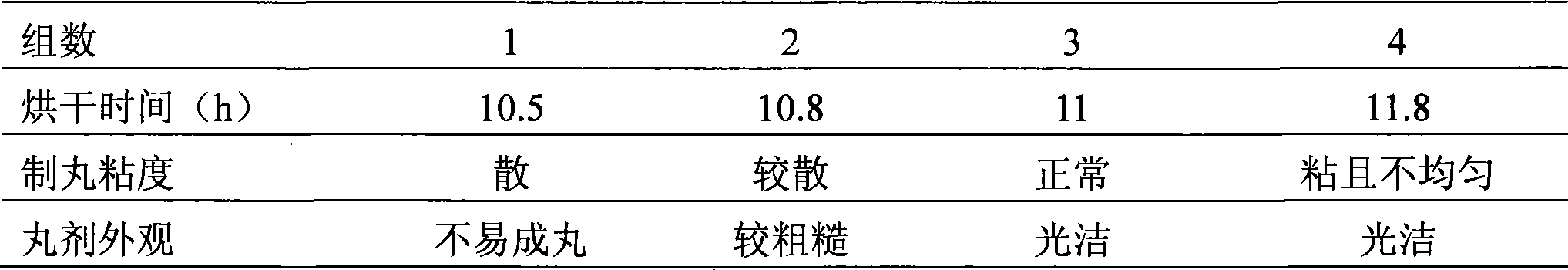
[0044] 2. Starch adding water test
[0045] Configure accordi...
experiment example 2
[0052] Experimental example 2 Experimental research on identification method
[0053] 1. Angelica identification test screening
[0054] (1) Preparation of test solution
[0055] Method 1: Take 3.85g of the original medicinal material equivalent to the pharmaceutical preparation of the present invention, grind it, add 20ml of ethyl ether, sonicate for 10 minutes, filter, evaporate the filtrate, add 1ml of ethanol to the residue to dissolve it, and use it as the test solution.
[0056] Method 2: Take 3.85g of the original medicinal material of the present invention, grind it, add 20ml of ethyl ether, sonicate for 20 minutes, filter, evaporate the filtrate, add 1ml of ethanol to the residue to dissolve it, and use it as the test solution.
[0057] Method 3: Take 3.85g of the original medicinal material of the present invention, grind finely, add 20ml of ethyl ether, sonicate for 30 minutes, filter, evaporate the filtrate, add 1ml of ethanol to the residue to dissolve it, and use it ...
experiment example 3
[0108] Experimental example 3 Check item experimental research
[0109] Research on heavy metals and arsenic salts
[0110] 1 Heavy metal inspection
[0111] Determination method: Take 2g of the fine powder of this product, place it in a crucible, carbonize at low temperature, and then completely ash at 600°C, add 2ml of hydrochloric acid to the residue, evaporate in a water bath, add 15ml of water, and check according to law (Chinese Pharmacopoeia 2005 Edition One Appendix IX E The second method). A total of three batches of samples were checked for heavy metal limits according to the determination method, and the content was less than 10 ppm, so they were not included in the text. The results are shown in Table 1.
[0112] Table 1 Heavy metal inspection results of three batches of samples
[0113]
[0114] 2 Arsenic salt inspection
[0115] test methods:
[0116] Preparation of standard arsenic spots: According to the first method under item F of Appendix IX F of the 2005 edit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com