Recombinant Kluyveromyces sp. expressing antibody or antibody analogue, and construction method and use thereof
An antibody analog, Kluyveromyces technology, applied to recombinant Kluyveromyces expressing antibody or antibody analog and its construction and application fields, can solve the problems of long construction period, cumbersome operation and high production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1, Expression of anti-HER2 humanized monoclonal antibody in Kluyveromyces lactis
[0052] 1. Obtaining the whole gene of anti-HER2 humanized monoclonal antibody
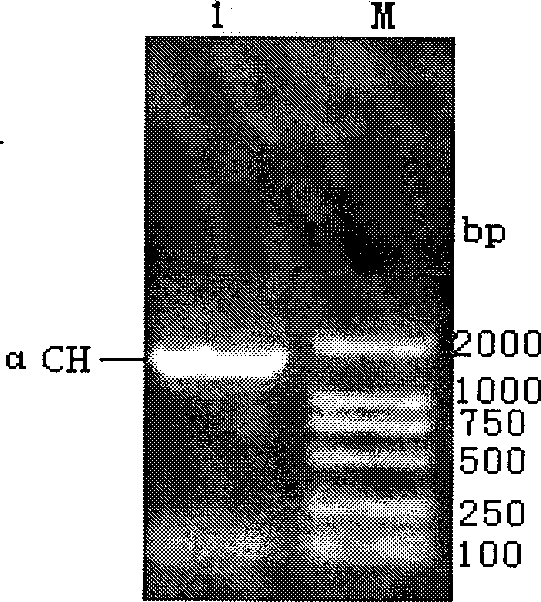
[0053]According to the light and heavy chain sequences of anti-HER2 humanized monoclonal antibodies reported on Genbank (Genbank: AAB49171.4 and AAB48814.1) and yeast preferred codons, synonymously mutate the commonly used enzyme cutting sites. Using the whole gene synthesis method, one-step PCR method to obtain antibody light chain gene (Light chain) L fragment (sequence 2 in the sequence listing) and heavy chain gene H (heavy chain) fragment (sequence 1 in the sequence listing), and with Alpha mating factor signal peptide.
[0054] For the one-step PCR method, refer to "Molecular Cloning Experiment Guide" (Second Edition, Science Press, 1995) by J. Sambrook et al. The PCR amplification conditions are as follows: after denaturation at 94°C for 5 minutes, 55 cycles of denaturation at 94°C for 30 sec,...
Embodiment 2
[0106] Example 2, Expression of anti-HER2 humanized monoclonal antibody in Kluyveromyces lactis variant
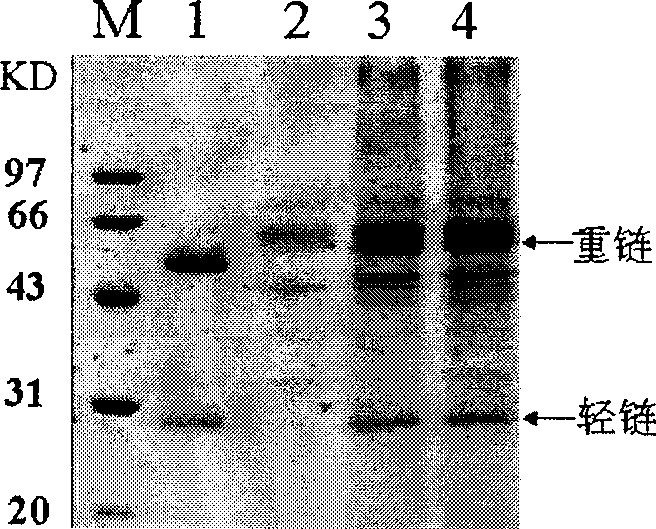
[0107] According to the method described in Example 1, the recombinant vector pYES2-och1-LAC4-CL and pPICZαA-ura3-LAC4-H were transformed into Kluyveromyces lactis KLGE02 CGMCC No.2400, and the recombinant vector pYES2-och1-LAC4-CL and pPICZαA-ura3-LAC4-H were transformed into K. lactis KLGE03CGMCC No.2401. Anti-HER2 monoclonal antibodies were expressed in K. lactis KLGE0 2CGMCC No.2400 and K. lactis KLGE03 CGMCC No.2401. Purified samples HER2-1 and HER2-2 were obtained according to the method of 4 in Example 1. Purified samples were subjected to 12% SDS-PAGE electrophoresis, and sent to the Instrument Center Laboratory of the Academy of Military Medical Sciences for N-terminal amino acid residue sequence analysis of the protein band of the heavy chain (methods refer to "Protein Technology Manual" edited by Wang Jiazheng and Fan Ming (Science Press, 2000)), the sequencin...
Embodiment 3
[0109] Example 3, Expression of Antibody Analog sTNFRII-IgGFc in Kluyveromyces lactis
[0110] 1. Fishing of sTNFRII gene and IgGFc gene
[0111] Take acid citrate dextrose (ACD) anticoagulated venous blood from healthy people, dilute it with Hanks' balanced salt solution (Invitrogen Corp. The 1640 medium (GIBCO, USA) of 10% fetal bovine serum (Hyclone Company, USA) adjusted the number of cells to be 5×10 6 cells / mL, placed in a cell incubator and incubated for 2 hours, then replaced with 1640 fresh medium containing lipopolysaccharide (LPS, 20 μg / mL) (Sigma) and 10% fetal bovine serum, continued to culture for 5 hours, and centrifuged to enrich the cells; TRIzol (Shanghai Sangon Bioengineering Technology Service Co., Ltd.) method to extract total RNA (operate according to the instructions of TRIzol); total RNA was subjected to RT-PCR to obtain cDNA (operate according to the instructions of the RT-PCR kit), and then use cDNA as a template , to amplify the human sTNFRII gene ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com