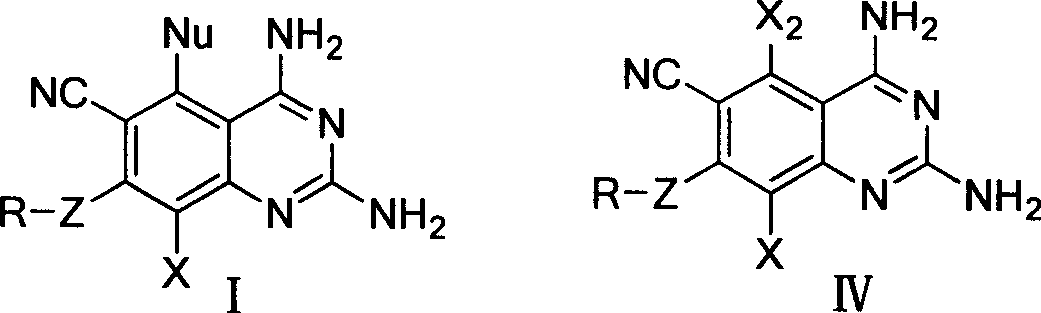
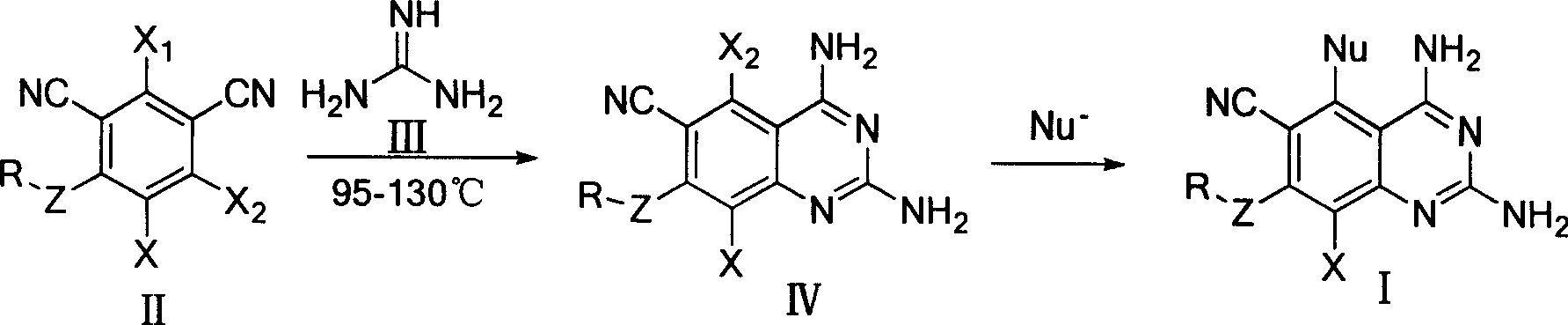
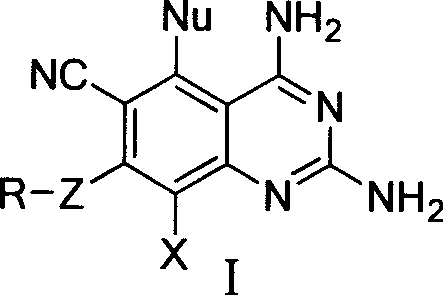
2,4-diamino quinazolines compounds and synthesis method thereof
A technology of diaminoquinazoline and synthesis method, applied in 2 fields, can solve problems such as difficult solvent, complicated post-processing process, environmental pollution of organic solvent and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Synthesis of 2,4-diamino-6-cyano-5,7,8-trichloroquinazoline (IV-A): Weigh 13.3 grams of 2,4,5,6-tetrachloro-1,3-metabenzene Diformonitrile (chlorothalonil) and 9.0 g of guanidine carbonate were thoroughly ground and stirred in a mortar, mixed evenly, and then placed in a constant temperature oven to react at 100°C. After the reaction was stable for 1 hour, the reactants were quickly ground, and after uniformity Increase the temperature to 115℃ and continue to heat the reaction in a constant temperature oven. The reactants gradually produce yellow products. The reactants will feel moist during the reaction (due to the decomposition of carbonic acid in guanidine carbonate to produce water). Grind the reaction every 1 hour during the reaction The reaction was continued after homogenization. After 3.5 hours of reaction, the raw material chlorothalonil was detected by TLC, and the reaction was complete. Take out the reactant and pour it into a beaker, stir and wash with a large ...
Embodiment 2
[0070] Synthesis of 2,4-diamino-6-cyano-5,7,8-trichloroquinazoline (IV-A): Weigh 1.0 g of 2,4,5,6-tetrachloro-1,3-metabenzene Diformonitrile (chlorothalonil) and 0.7 g of guanidine carbonate were thoroughly ground and stirred in a mortar, mixed evenly, and placed in a microwave reactor. The temperature was adjusted to 120°C. After microwave irradiation for 30 minutes, TLC detection, raw material All chlorothalonil disappeared and the reaction was complete. Take out the reactant and pour it into a beaker, stir and wash with a large amount of water, and then filter with suction. During the pumping rate, the reactant is washed with water repeatedly to remove all the remaining guanidine carbonate in the reactant. After washing with water, wash with a small amount of dichloromethane. The reactant, wash away the very small amount of chlorothalonil that may be left in the reactant. After washing, the product was drained to obtain 1.0 g, the yield was 89.9%, and the melting point of the p...
Embodiment 3
[0072] Synthesis of 2,4-diamino-6-cyano-5,7-dichloro-8fluoroquinazoline (IV-B): Weigh 5-chloro-2,4,6-trifluoro-1,3 21.7 g of isophthalonitrile and 27.3 g of guanidine carbonate were thoroughly ground and stirred in a mortar. After mixing, they were placed in a constant temperature oven for reaction at 85°C. After 1 hour of stable reaction, the reactants were quickly ground, and the temperature was raised after uniformity. Continue to heat and react in a constant temperature oven at 105°C, and the reactants gradually generate orange-yellow products. During the reaction, the reactants were ground once every 40 minutes, and the reaction was continued after homogenization. After the reaction was heated for 3 hours, the TLC detection showed that all the raw materials of chlorothalonil disappeared and the reaction was complete. Take out the reactant and pour it into a beaker, stir and wash with a large amount of water, and then filter with suction. In the process of suction, the reactan...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com