Sustained release tablets containing cefaclor active component and preparation method thereof
A technology of cefaclor and active ingredients, which is applied in the field of cefaclor sustained-release tablets and its preparation, and can solve the problems of inconvenient medication for patients, large side effects, and unstable blood drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
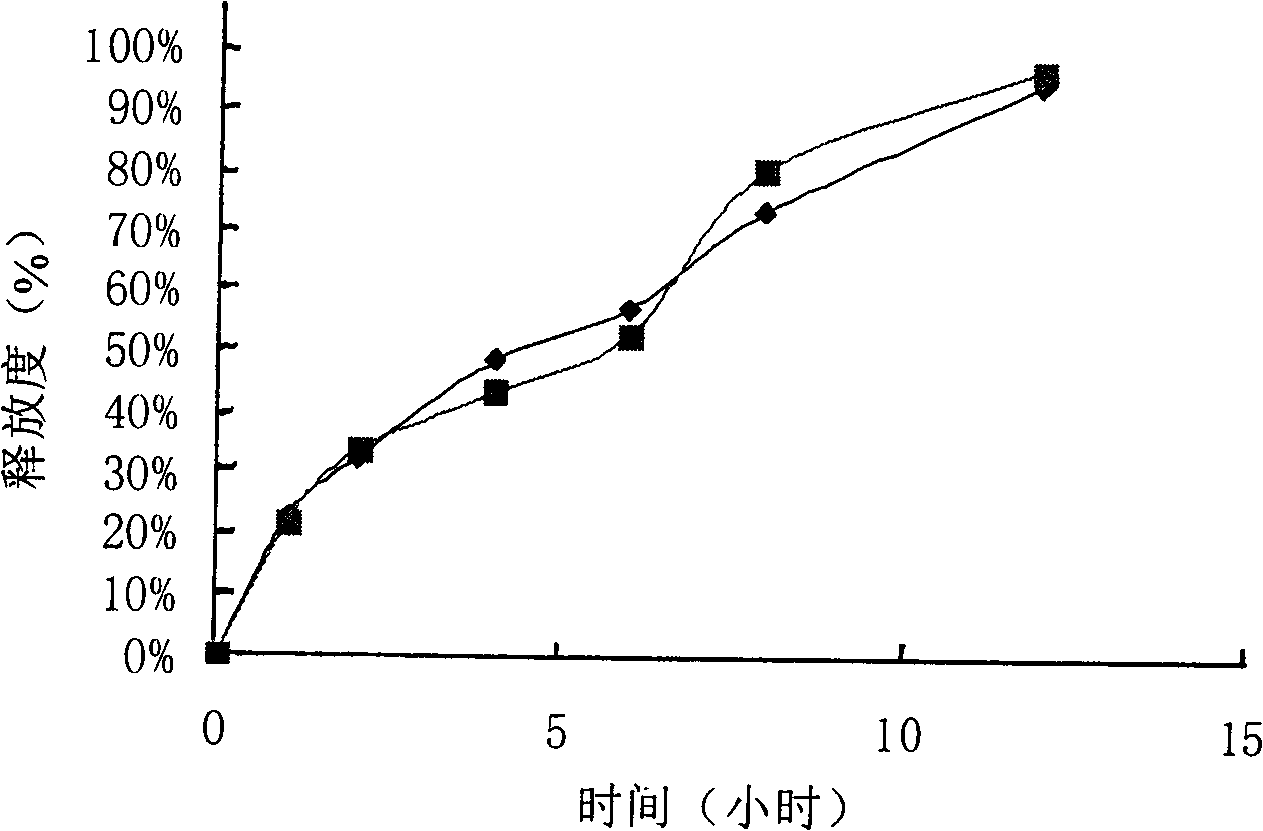
example 1
[0107] Tablet prescription:
[0108] Specification: 125mg
[0109] Product name Quantity per tablet
[0110] Cefaclor 125.00mg
[0111] Hypromellose (4000 and 100,000 grades) 13.00mg
[0112] Acrylic resin No. II 3.00mg
[0113] Lactose 13.00mg
[0114] 75% ethanol solution 75.0ml
[0115] Magnesium Stearate 3.00mg
[0116] Total: 157.00mg
[0117] Coating prescription:
[0118] Specification 125mg
[0119] Product name Quantity per tablet
[0120] Hypromellose (4000 and 100,000 grades) 8.00mg
[0121] Titanium Dioxide 2.00mg
[0123] Tween-80 0.001ml
[0124] Diethyl phthalate 0.002ml
[0125] Preparation ethanol 134.40mg
[0126] Total: 12.000mg
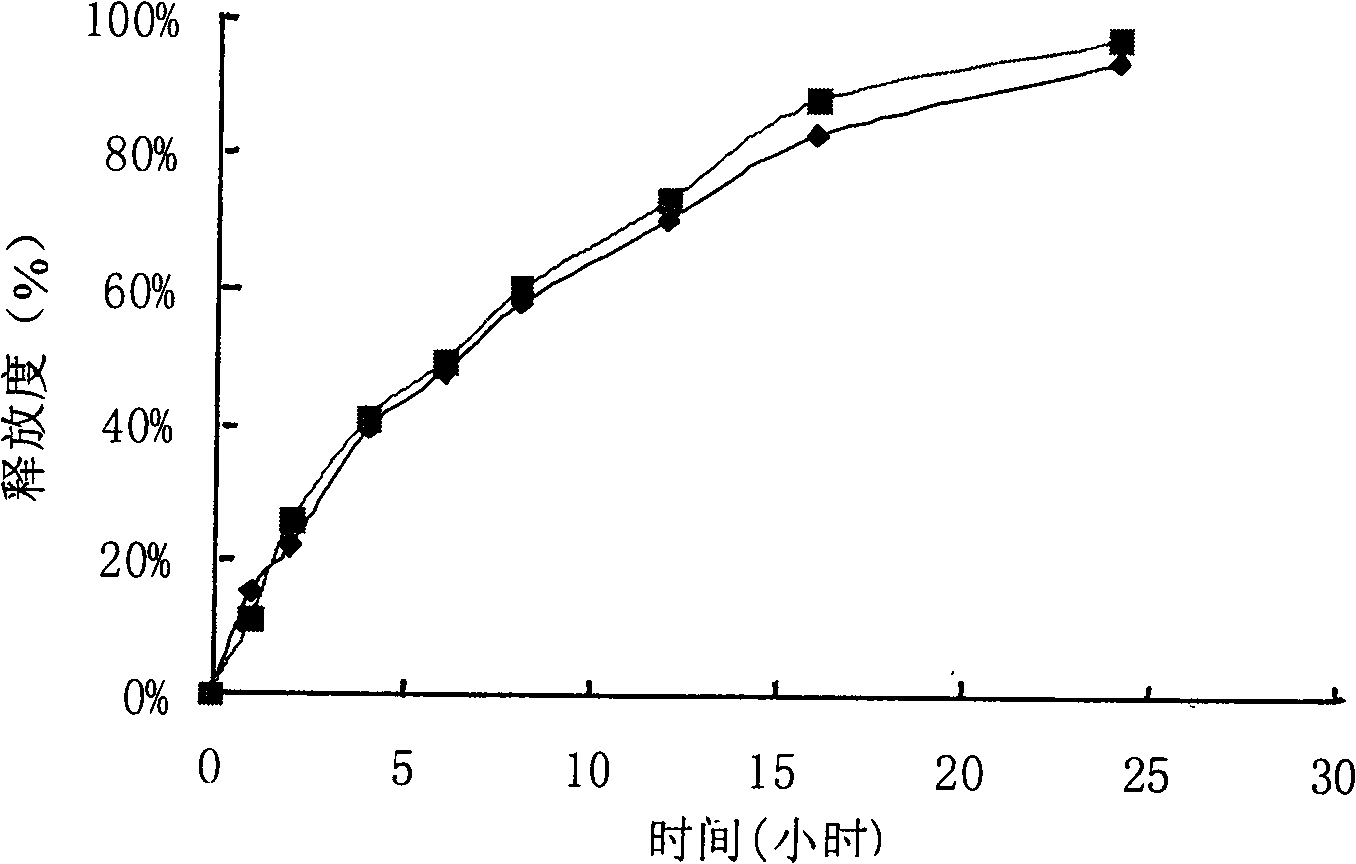
example 2
[0128] Tablet prescription:
[0129] Specification: 125mg
[0130] Product name Quantity per tablet
[0131] Cefaclor 125.00mg
[0132] Hypromellose (grade 100,000) 13.00mg
[0133] Acrylic resin No. II 3.00mg
[0134] Lactose 13.00mg
[0135] 75% ethanol solution 75.0ml
[0136] Magnesium Stearate 3.00mg
[0137] Total: 157.00mg
[0138] Coating prescription:
[0139] Specification 125mg
[0140] Product name Quantity per tablet
[0141] Hypromellose (grade 100,000) 8.00mg
[0142] Titanium Dioxide 2.00mg
[0144] Tween-80 0.001ml
[0145] Diethyl phthalate 0.002ml
[0146] Preparation ethanol 134.40mg
[0147] Total: 12.000mg
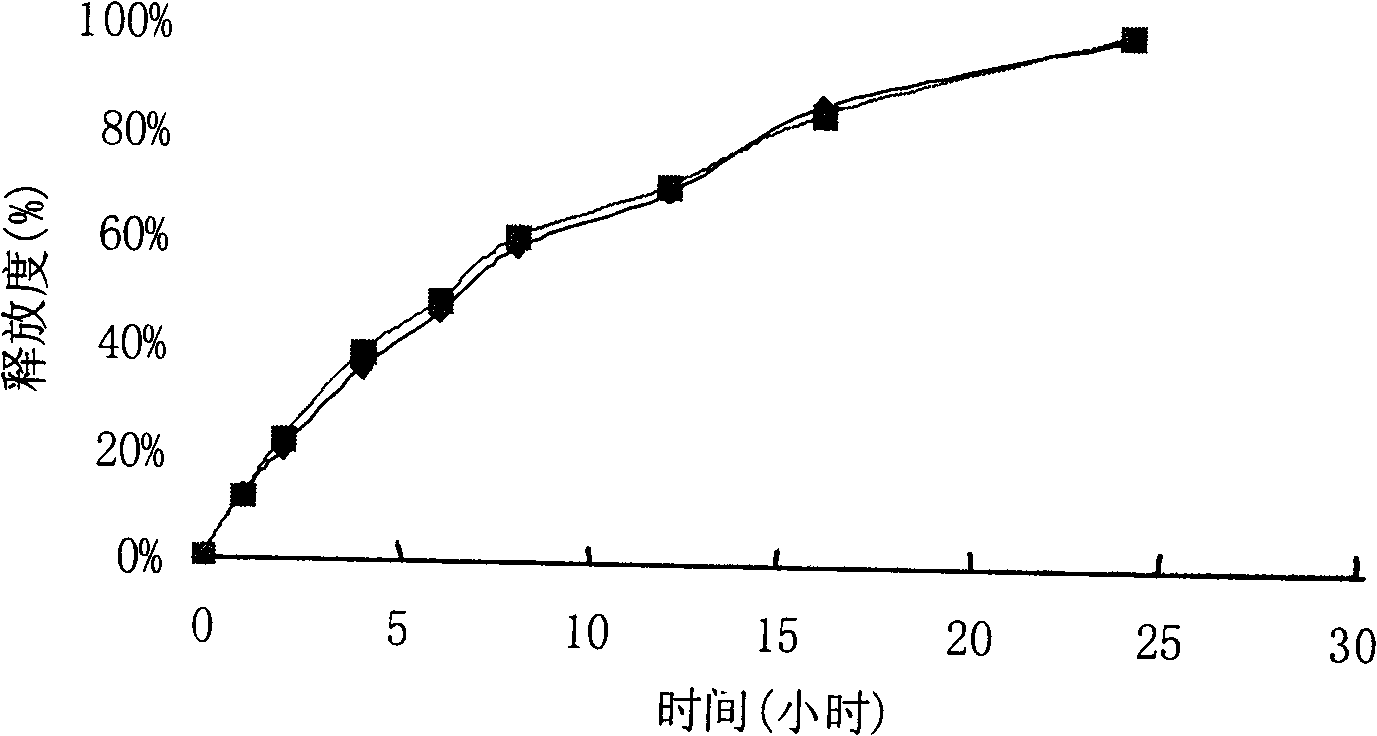
example 3
[0149] Product name Quantity per tablet
[0150] Cefaclor 375.00mg
[0151] Hypromellose (grade 100,000) 40.00mg
[0152] Acrylic resin No. II 6.00mg
[0153] Lactose 80.00mg (60.00mg inside; 20.00mg outside)
[0154] Povidone K30 8.00mg
[0155] 50% ethanol solution 18.00mg
[0156] Magnesium Stearate 8.00mg
[0157] Total 517.00mg
[0158] Coating prescription:
[0159] Strength 375mg
[0160] Product name Quantity per tablet
[0161] Hypromellose (grade 100,000) 24.00mg
[0162] Titanium Dioxide 6.00mg
[0164] Tween-80 0.003ml
[0165] Diethyl phthalate 0.005ml
[0166] Preparation Ethanol 400.00mg
[0167] Total: 36.000mg
[0168] The preparation technology of 1-3 embodiment:
[0169] (1) Processing of raw and auxiliary materials: Cefaclor, hypromellose, acrylic resin II, and lactose were pulverized by a universal pulverizer.
[0170] (2) Pre-mixing: Add lactose, hydroxypropyl methylcellulose, acrylic resin No. II, and cefaclor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com