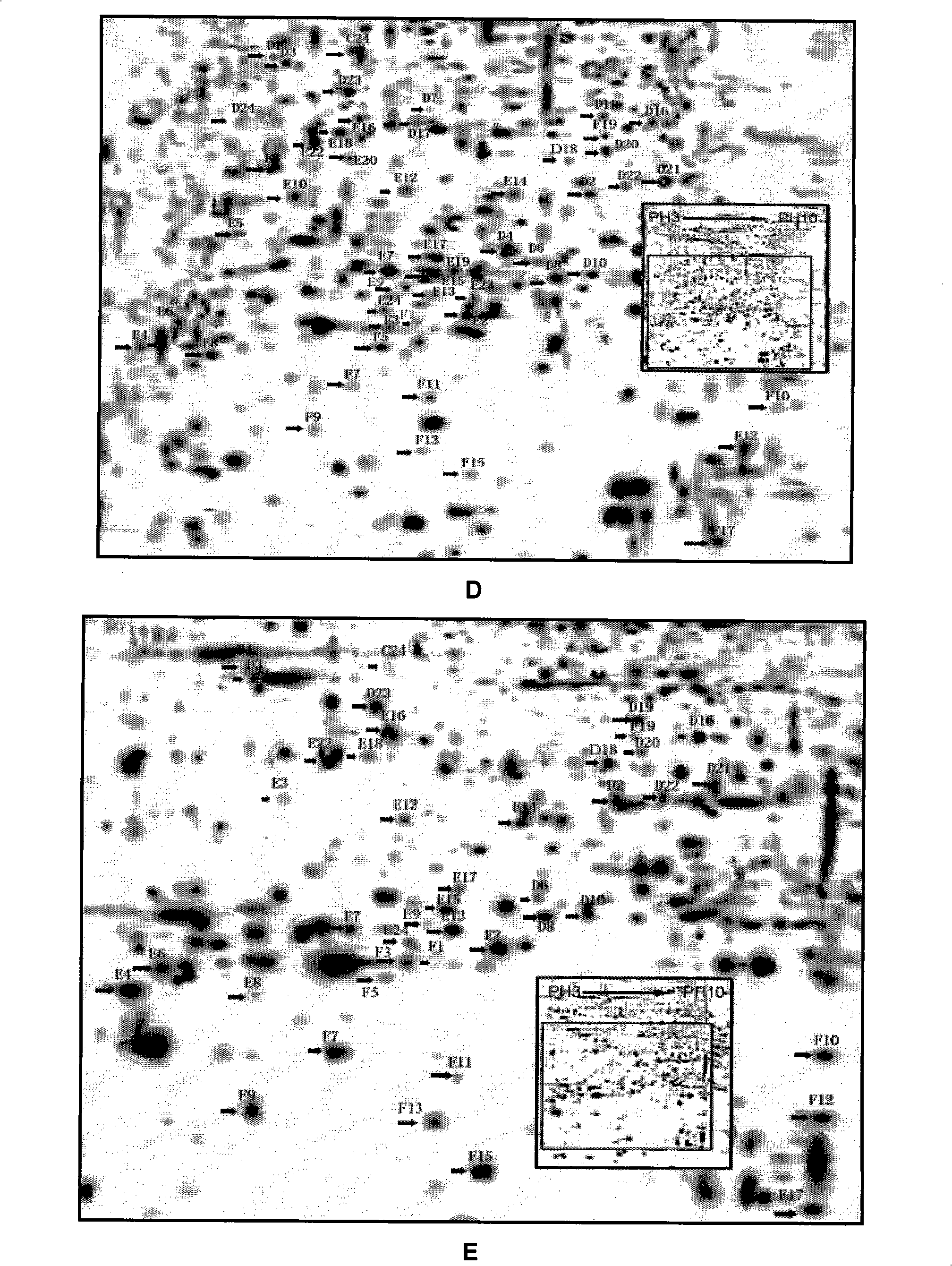
Construction and application of cardiac muscle cell protein difference expression atlas
A cardiomyocyte differential expression technology, applied in the field of constructing the protein differential expression map of icariin-induced stem cell-directed differentiation cardiomyocytes, can solve the problems that have not yet been studied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Construction of differential protein expression map of ICA-induced directed differentiation of stem cells into cardiomyocytes
[0023] (1) To establish a model of drug-induced stem cell directed differentiation into cardiomyocytes: ES cells were cultured in hanging drops to form embryoid bodies, and drug icariin was added to induce their directed differentiation into cardiomyocytes.
[0024] (2) Preparation of two-dimensional electrophoresis protein samples: collect the above-mentioned differentiated cells in different differentiation phases, rinse with phosphate buffer, blot the residual liquid, add lysis buffer and mix well, freeze and thaw repeatedly in liquid nitrogen for 3 times, add 50 μg / ml RNase and 200 μg / ml DNase, place at 4°C for 15 minutes, centrifuge at 16100 rpm, discard the supernatant, wash twice with 1% dithiothreitol (DTT) pre-cooled acetone, dry at room temperature for 1 hour, and dissolve the hydration solution The protein concentration wa...
Embodiment 2
[0029] Example 2 ICA Induced Directed Differentiation of Mouse ES Cells into Cardiomyocytes in Vitro
[0030] 1. Differentiation culture of ES cells induced by ICA
[0031] (1) Hanging drop for three days
[0032] Undifferentiated ES cells were made into single-cell suspension with ES cell differentiation medium, counted, and diluted to 2.0×10 with high-glucose DMEM medium containing 20% fetal bovine serum. 4 cells / ml, 30 μl / drop of cell solution (each hanging drop contains 600 ES cells) was added dropwise on the inner surface of the culture dish cover for hanging drop differentiation and cultured for 3 days until embryoid bodies (embryonic bodies, EBs) were formed.
[0033] (2) Suspension for two days
[0034]The bottom of the culture dish was paved with 1% agar solution in advance, and the formed EBs were transferred to a culture dish containing high-sugar DMEM and 20% fetal bovine serum medium for suspension for 2 days.
[0035] (3) adherent culture
[0036] Transfer ...
Embodiment 3
[0040] Example 3 Two-dimensional gel electrophoresis separation of protein expression points of ICA-induced cardiomyocyte differentiation of ES cells
[0041] Isoelectric focusing electrophoresis is performed first to separate the total protein according to the isoelectric point, and then SDS-PAGE is further separated according to the molecular size. The electrophoretic map obtained by staining is a two-dimensional distribution of protein maps. The target protein was discovered and determined by analyzing the protein expression changes of ES cells differentiated into cardiomyocytes at different differentiation stages and after ICA induction by comparing protein maps.
[0042] (1) Sample preparation
[0043] A. Cell samples
[0044] Aspirate the culture solution, and collect the cells (spontaneously differentiated and differentiated cardiomyocytes induced by ICA for 3 days and 7 days in adherent culture) with a cell scraper. Add D-Hanks solution, centrifuge at 1500 rpm for 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com