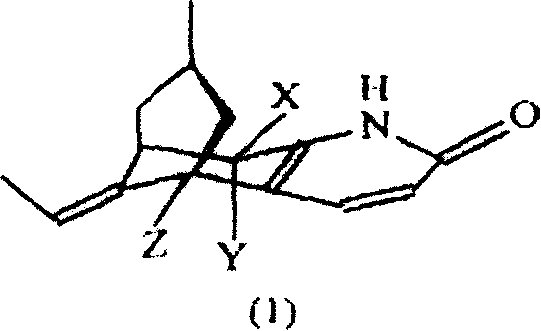
Huperzine and its derivant or its salt implantation agent, its preparation method and application
A technology of huperzine A and an implant is applied in the directions of medical preparations containing active ingredients, drug delivery, and drug combinations, which can solve the problems of inability to take medicines on time, low bioavailability, etc. The preparation process is simple and the effect of reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
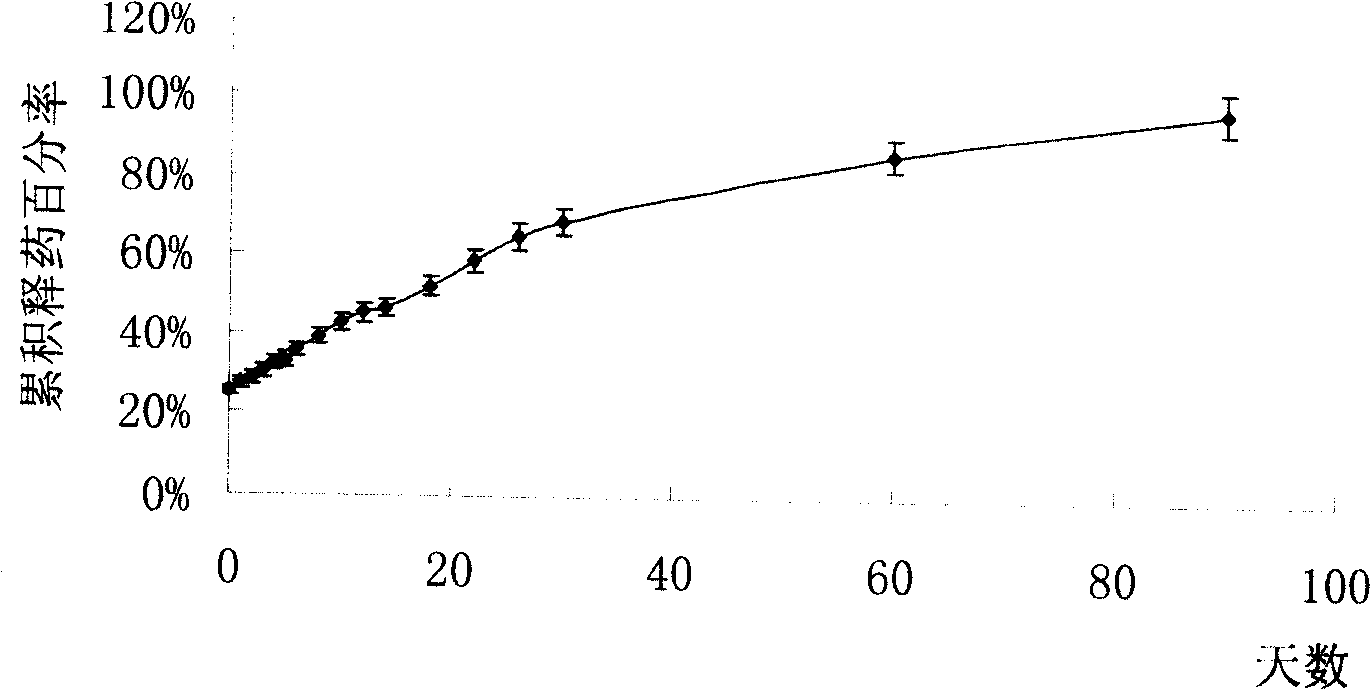
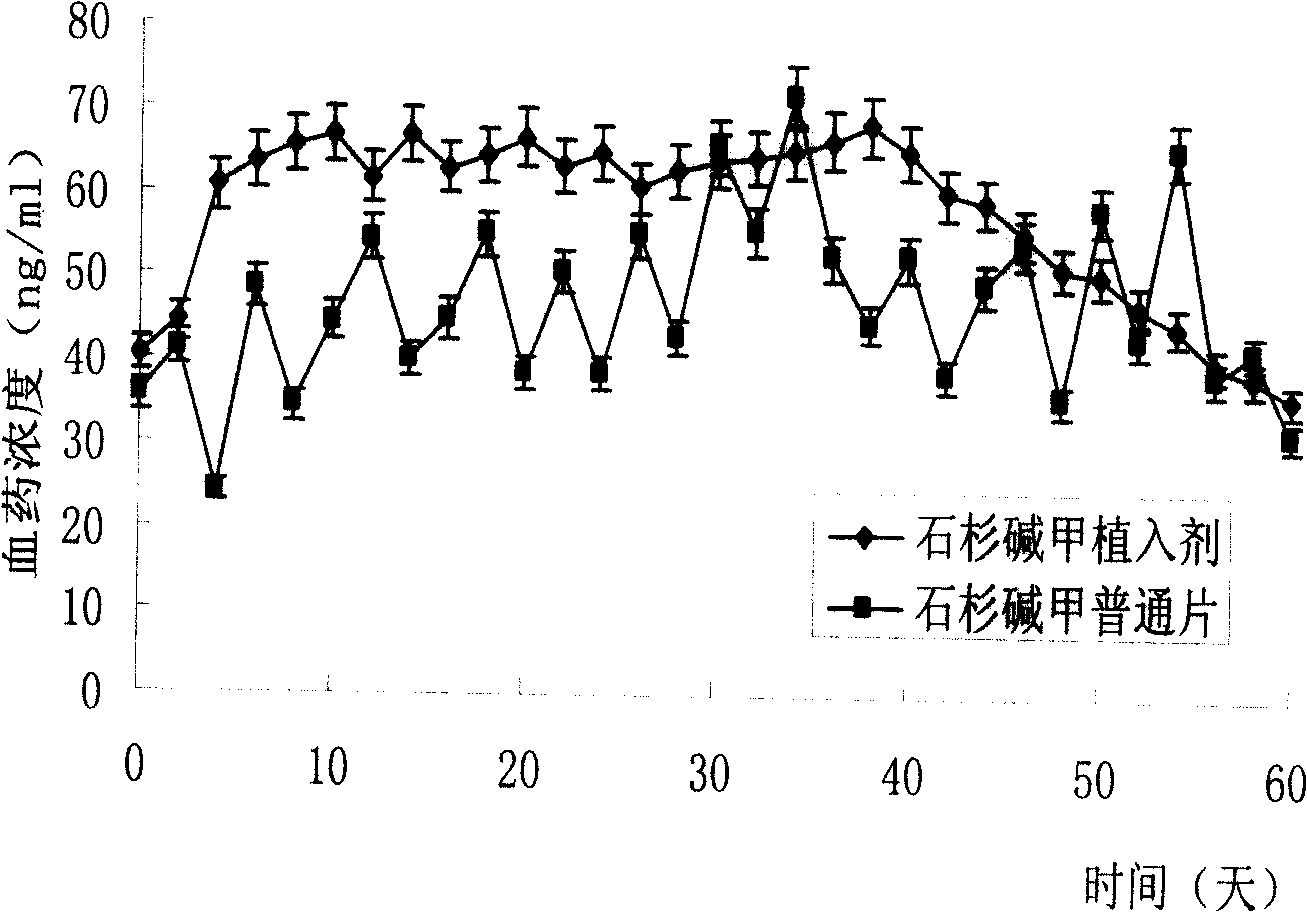
[0048] A long rod-shaped mold was prepared with dimethylsiloxane (PDMS), and the PDMS mold was soaked in acetone for 0.5 h to fully infiltrate the surface. Dissolve 18g of PLGA in 100ml of acetone at 30°C, pour this solution on the PDMS mold, dry at 60°C for 2 hours, and after cooling sufficiently, peel off the PDMS mold, take out the PLGA cavity, and add 150mg of Huperzine A. At both ends of the cavity, 1g of PLGA film was taken and sealed by heat and pressure to form a solid huperzine A implant. Check its tightness with conductometric method. That is, put the drug-containing implant into quantitative deionized water, and detect the change of the current value after stirring. If the current value increases to a fixed value after the implant is placed, it indicates that the device leaks medicine and is a substandard product.
Embodiment 2
[0050] Soak PDMS with acetone for 0.5h to fully infiltrate the surface and use it as a rod-shaped mold. Dissolve 10g of PLA and 15g of PLGA in a mixed solvent of 100ml of acetone and 20ml of dichloromethane at 30°C, pour this solution on the PDMS mold, dry at 60°C for 2 hours, and after fully cooling, peel off the PDMS mold and take out the PLGA cavity , Add 200mg huperzine A. At both ends of the cavity, 4g of PLGA film was hot-pressed and encapsulated to make a solid huperzine A implant. And use the conductometric method to test its tightness.
Embodiment 3
[0052] Soak PDMS with acetone for 1h to fully infiltrate and use it as a rod-shaped mold. Dissolve 20g of PLA and 10g of PLGA in a mixed solvent of 100ml chloroform and 80ml tetrahydrofuran at 30°C, pour the solution on a PDMS mold, dry at 45°C for 6 hours, and after cooling fully, peel off the PDMS mold, take out the PLGA cavity, and add 600mg Huperzine A. At both ends of the cavity, 9g of PLGA film was hot-pressed and encapsulated to make a solid huperzine A implant. And use the conductometric method to test its tightness.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
| Intrinsic viscosity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com