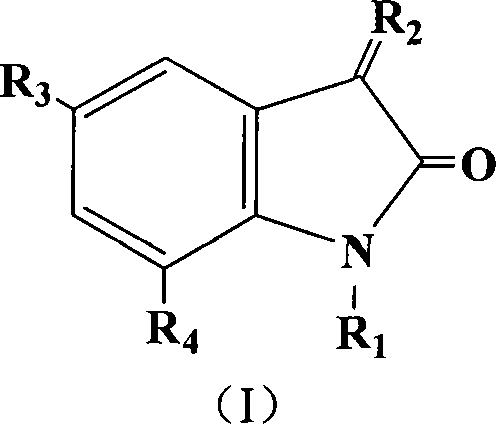
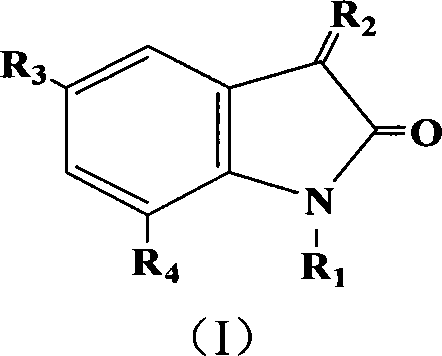
Acetolactate synthetase AHAS restrainer combination
A technology of acetolactate and composition, applied in the field of small molecule inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
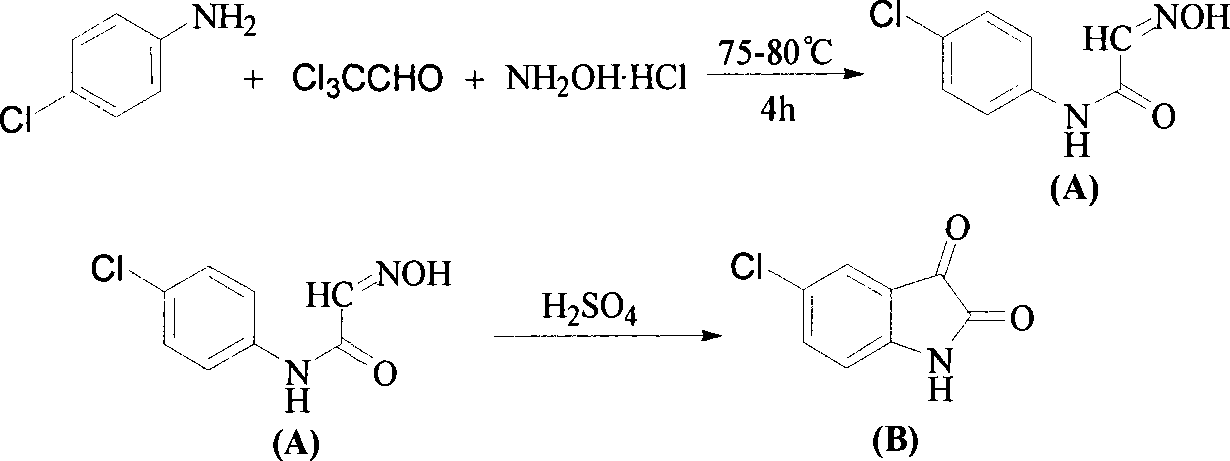
[0020] Embodiment 1: the preparation of 5-chloro-indole-2,3-dione
[0021]
[0022] Add 4.0ml (40mmol) of chloral hydrate and 45.9g (324mmol) of anhydrous sodium sulfate to 120mL of water, raise the internal temperature to 40°C, stir until clear, and prepare the prepared aqueous solution of p-chloroaniline hydrochloric acid [p-chloroaniline 4.59g ( 36.0mmol) dissolved in 54mL 2.5% hydrochloric acid] was added dropwise to the reaction solution, and the addition was completed in 10-15 minutes. The reaction was continued for 0.5h, and a large amount of white precipitate was precipitated. At this time, 7.5g (108mmol) of hydroxylamine hydrochloride was quickly added, and the temperature was raised to 75°C , reacted for 4 hours, cooled to room temperature, suction filtered, and dried to obtain 6.78 g of khaki-colored 4-chloro-1-isonitroso-acetanilide (A) powder, with a yield of 95%.
[0023] Preheat 40ml of concentrated sulfuric acid to 75°C, add 6.00g (30mmol) of 4-chloro-1-ison...
Embodiment 2
[0025] Example 2: Inhibition of AHAS enzymes
[0026] The Arabidopsis AHAS gene (pET-ATAHAS: T86-CHis) was overexpressed in Escherichia coli (BL21(DE3)), and pure AHAS was obtained after IMAC purification by immobilized metal affinity chromatography (Chang A.K., et al.Biochem.J. , 327, 161-169). When measuring the activity, the pH value of the reaction solution is 7.0, the volume is 250 μL, and contains 50 mM Na 2 HPO 4 / NaH 2 PO 4Buffer system, 50mM sodium pyruvate, 10mM magnesium chloride, 1mM thiamine diphosphate (ThDP), 10μM flavin adenine dinucleotide (FAD), 100μg / mL test compound, react at 37°C Initiate for 30 min, add 25 μL of 10% HO 2 SO 4 Stop responding. Heat the system at 60°C for 15 min to convert all the acetolactate generated into 3-hydroxy-2-butanone, then add 250 μL of 0.5% creatine and 250 μL of 5% α-naphthol (in 4M NaOH), in Reheat at 60°C for 15 minutes, cool for 15 minutes, read the absorbance at 525nM, and calculate the inhibition of chlorine with ...
Embodiment 3
[0027] Example 3: Inhibition of Rapeseed Root Length
[0028] The test target is rapeseed (Brassica napus). Spread a piece of filter paper with a diameter of 5.6 cm in a petri dish with a diameter of 6 cm, add 2 ml of a 100 μg / mL or 10 μg / mL solution of the test compound (Table 1), repeat each concentration twice, and set clear water as a control. Sow 15 rapeseeds soaked for 4-6 hours, and measure the length of the embryo after culturing in the dark at 30±1°C for 48 hours. Compared with the blank control, the percentage inhibition was calculated. Summarized in Table 1. Table 1 shows the molecular structure and in vivo and in vitro biological activity test results of the series of compounds.
[0029] Table 1 Biological activity data of a series of compounds
[0030]
[0031]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com