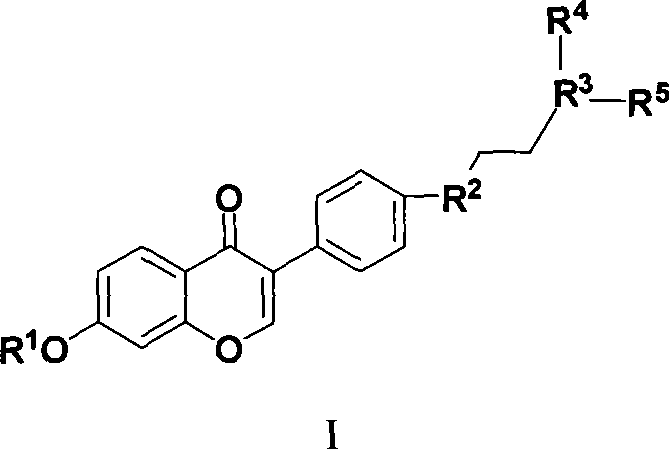
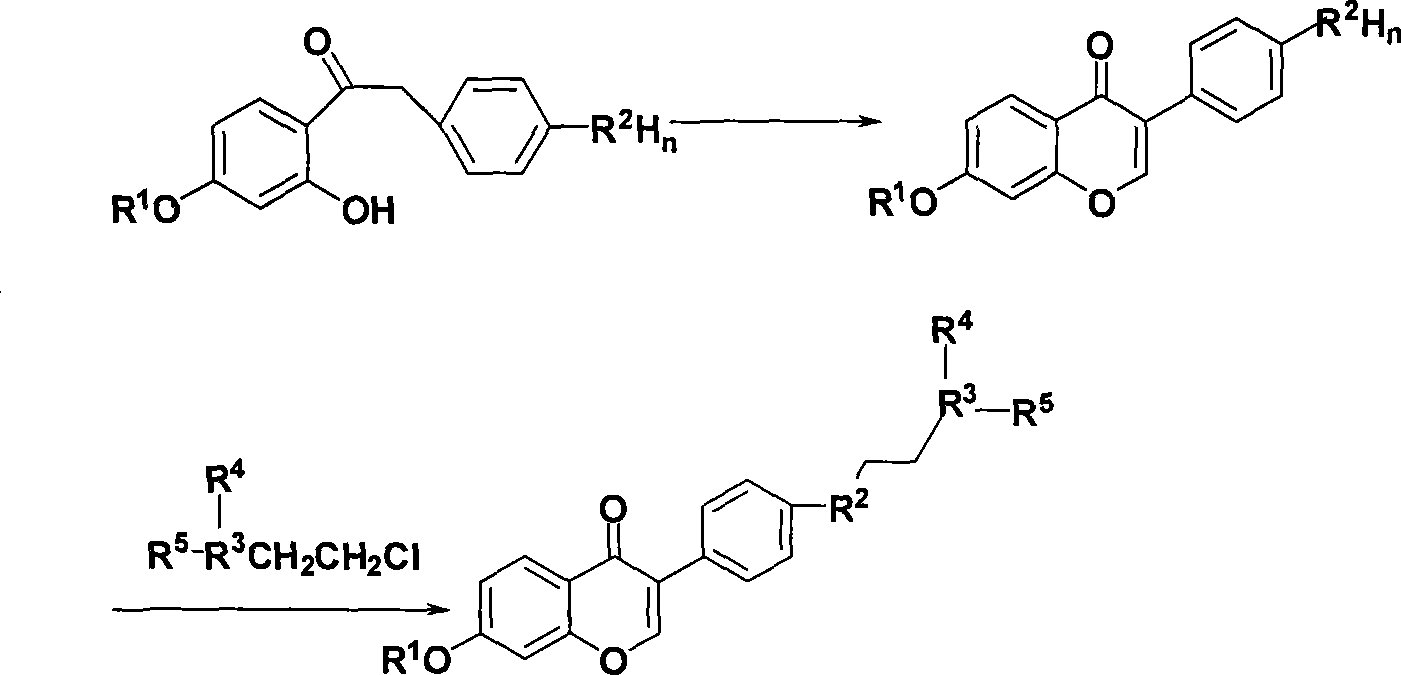
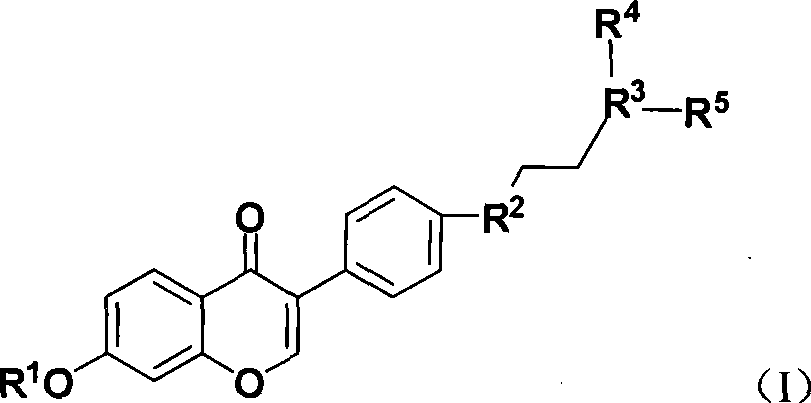
Benzopyrone derivative, preparation method and medical use thereof
A pharmaceutical and compound technology, applied in the field of benzopyrone derivatives to treat ER-type breast cancer, can solve the problems of patients losing fertility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Preparation of m-methoxyphenol (III)
[0059] Dissolve 13.2g of resorcinol equivalent to 0.12mol in 100ml of toluene, add a solution made of 0.5g of tetrabutylammonium bromide, 6.0ml of water and 28.0ml of 5N NaOH, heat up to 50°C, stir and drop Add 13.3ml of dimethyl sulfate equivalent to 0.14mol, and continue to keep warm for 2h. The pH was adjusted to neutral with acetic acid, and the separated aqueous phase was extracted with 30 ml of toluene. The organic phases were combined, washed with water and saturated sodium chloride solution, dried over anhydrous sodium sulfate, distilled under reduced pressure, and the solvent was evaporated to obtain a crude compound III in the form of light yellow liquid, which was directly used in the next reaction.
Embodiment 2
[0061] Preparation of 2-hydroxy-4-methoxy-4'-hydroxydeoxybenzoin (IV)
[0062] Add 9.0 g of p-hydroxyphenylacetic acid corresponding to 0.059 mol, 0.6 g of p-toluenesulfonic acid and 36 ml of freshly steamed boron trifluoride-ether solution to the above system compound III, and react at 80° C. for 6 h. After cooling, slowly pour into 150ml of saturated sodium bicarbonate solution, stir vigorously, filter out the precipitated solid, recrystallize with absolute ethanol, and decolorize with activated carbon to obtain 7.52g of off-white powder with a yield of 48.6%. m.p.150-152°C.
Embodiment 3
[0064] Preparation of 7-methoxydaidzein (V)
[0065] Dissolve 6.0g of compound IV equivalent to 0.023mol, 8.0g of N,N-dimethylacetamide dimethyl acetal (DMF-DMA) equivalent to 0.067mol into 20ml of DMF, heat to reflux for 4h, and cool Then pour it into 100ml of ice water, stir, filter out the precipitated solid, and recrystallize from methanol to obtain 5.11g of light yellow powder with a yield of 82%. m.p.215-218°C. ESI-MSm / z: 267[M-H] - .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com