Xanthiphenylketamine crystal and its preparation method and medical use
A technology of piperphentonamine and crystals, which is applied in the fields of piperphentonamine crystals and its preparation and medicinal uses, and can solve problems such as piperphentonamine crystals not specified, preparation methods of piperphentonamine crystals not specified, and medicinal uses, etc. problems, to achieve the effect of good storage stability, high purity and high biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Take 1.56g of piperphentonamine hydrochloride (see Chinese patent 02125318.8 for the preparation method of piperphentonamine hydrochloride) and put it into a 500ml eggplant-shaped bottle, add 100ml of ethyl acetate, and slowly add saturated aqueous sodium bicarbonate solution under cooling in an ice-water bath 80ml, the solution gradually changed from a suspension to a clear solution with the addition of saturated aqueous sodium bicarbonate solution, and after one hour, the solution turned into a cloudy solution again, and was stirred for 5 hours under ice-water bath cooling, the organic layer was separated, and the water layer was re- Extracted with ethyl acetate, the combined organic layers were washed with NaCl saturated aqueous solution, washed with anhydrous NaCl 2 SO 4 Dry, filter, and when the filtrate is concentrated to a small amount of liquid, off-white flaky crystals are precipitated, filtered, off-white flaky crystals are washed with absolute ethanol, dried ...
Embodiment 2
[0039] Take 720 mg of piperphentonamine-like white flaky crystals described in Example 1, heat and dissolve them with 90 ml of ethyl acetate to obtain a colorless and clear solution, filter, and place the filtrate in an incubator at 8° C. for 10 days to slowly precipitate out the like White flaky crystals are piperphentonamine crystal grains. When the grains gradually become larger and thicker and grow into single crystals, they can be used as single crystal X-diffraction samples for X-ray diffraction of piperphentonamine single crystals. test.
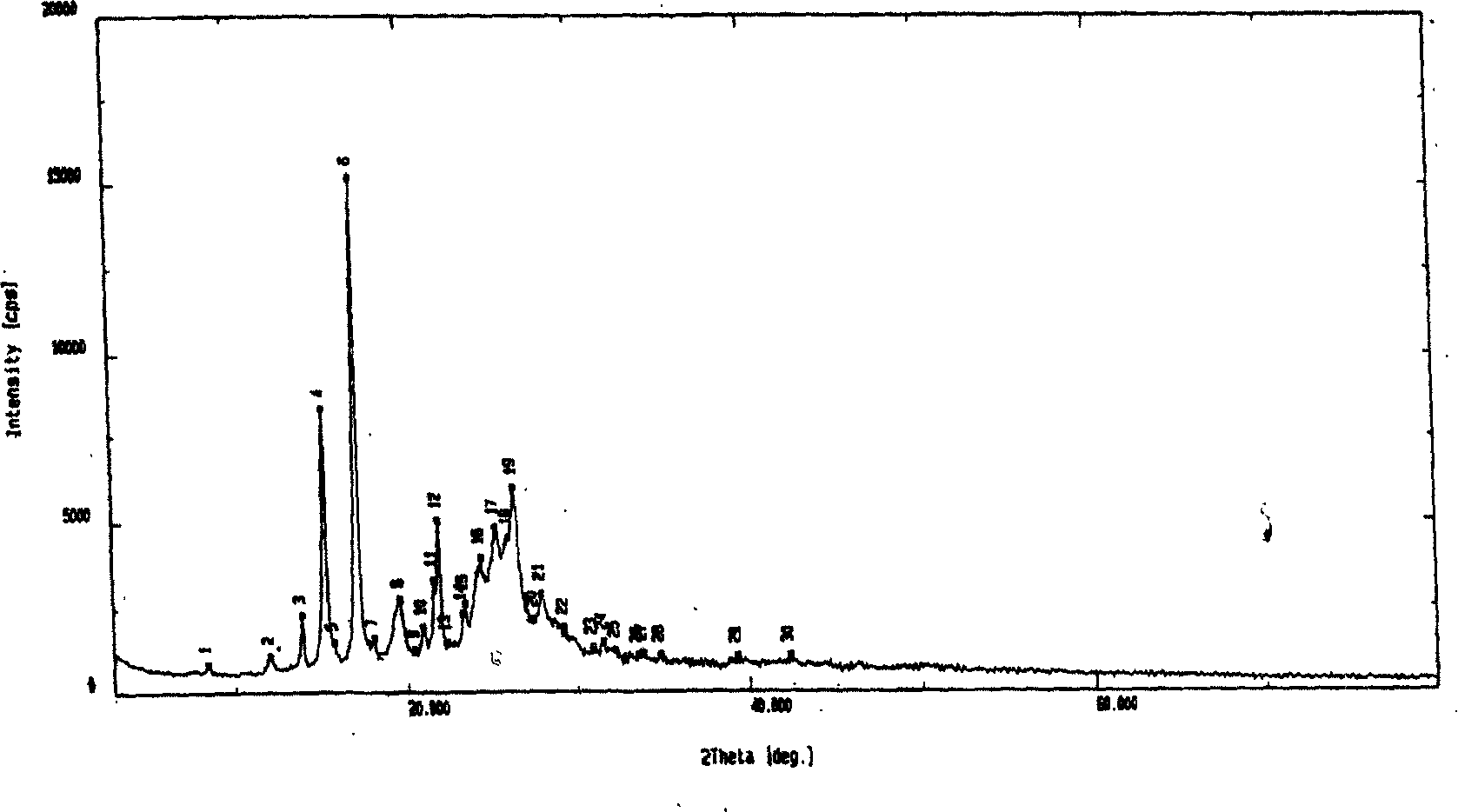
[0040] Instrument model: Japan MAC DIP-2030K single crystal X-ray surface detector. Test conditions: moka radiation, graphite monochromator, distance between crystal and IP plate d=100mm, tube pressure 50Kv, tube current 90mA, ω scan, maximum 20 angle is 50.0°, scan range is 0-180°, swing angle is 5°, the interval is 5°, the scanning speed is 1.5° / min, each frame is scanned twice, and a total of 3 images are taken, with 3870 independ...
Embodiment 3
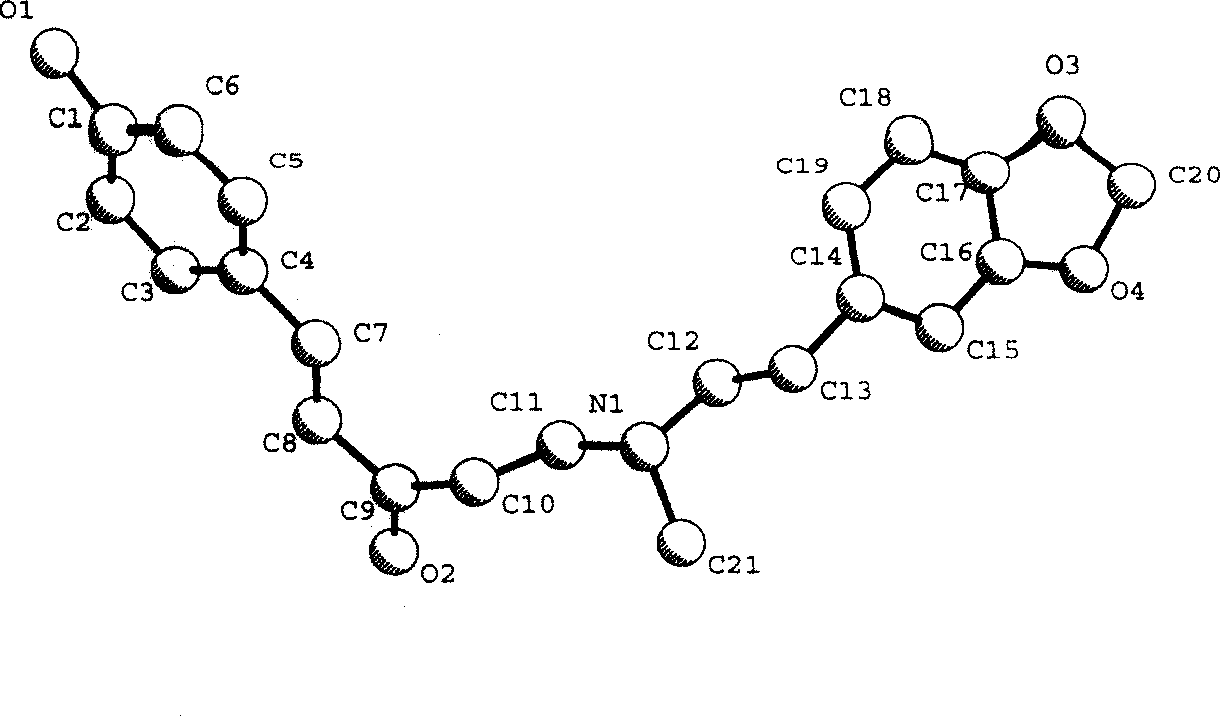
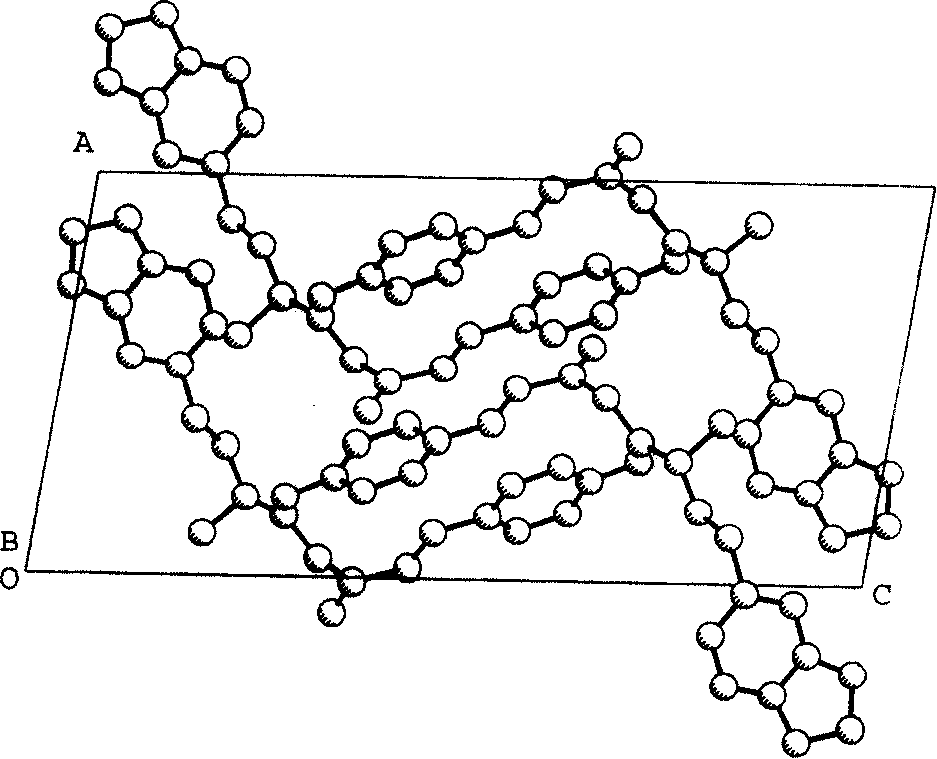
[0046] Analyze the crystal structure with the direct method on the microcomputer, directly obtain 24 non-hydrogen atom positions from the E map, use the least square method and the difference Fourier method to obtain the remaining non-hydrogen atom positions, and use the least square method to correct the structural parameters and discrimination Atomic types, use the geometric calculation method and difference Fourier method to obtain all hydrogen atom positions, the final reliability factor Rf=0.069, Rw=0.063 (w=1 / σ|F| 2 ). Finalize the stoichiometric formula as C 21 h 23 o 4 N 1 , the calculated molecular weight is 353.42, and the calculated crystal density is 1.261g / cm 3 . The molecular structure diagram of the compound is shown in the description, figure 2 is the projection diagram of the three-dimensional structure of the molecule shown, image 3 Shown is the unit cell packing diagram of the molecules along the b-axis. Table 2 shows the atomic coordinate paramete...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com