Diarylhydantoin compounds
A technology of compounds and mixtures, applied in the field of diarylhydantoin compounds, can solve problems such as time-consuming
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
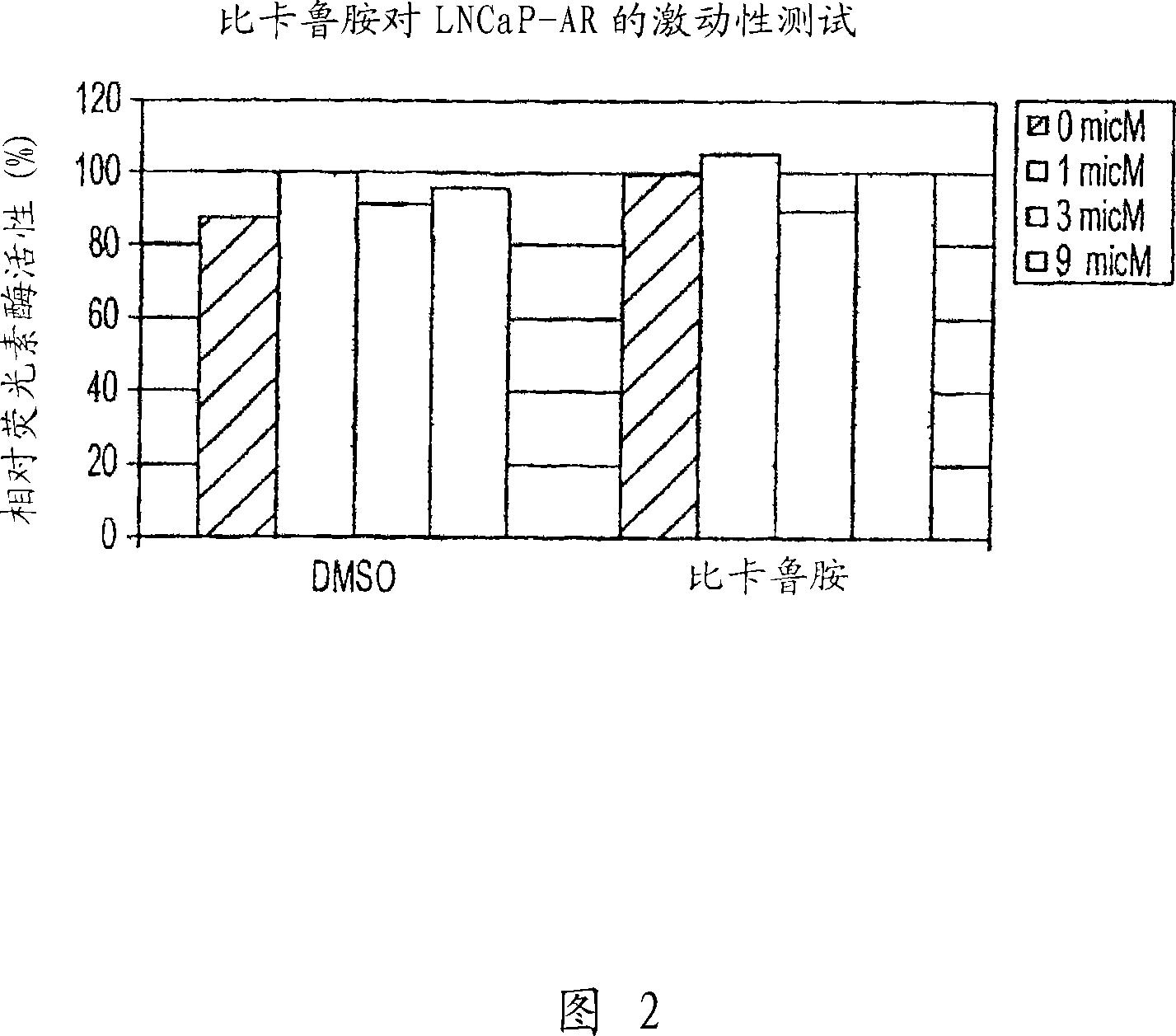
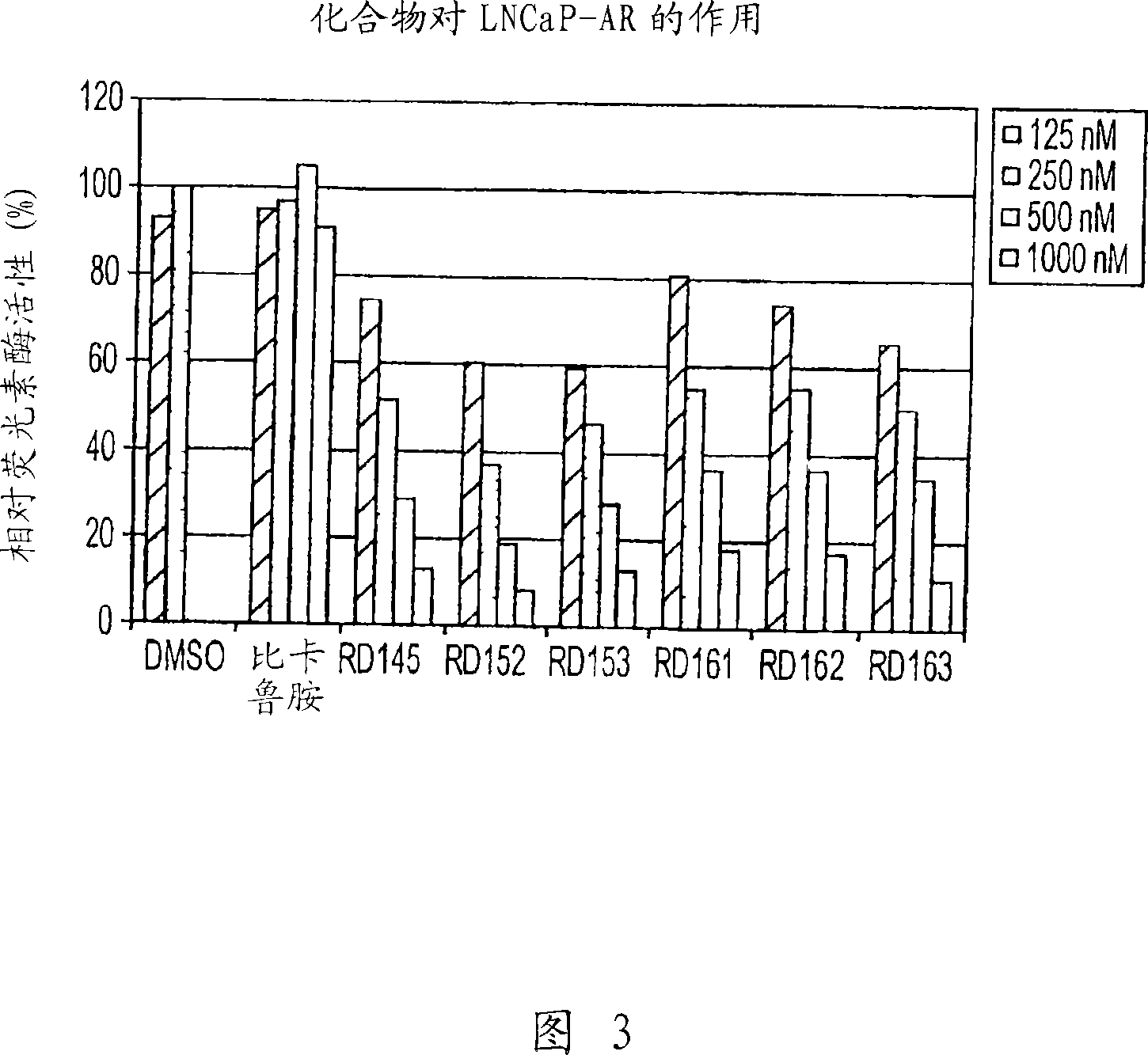
Image
Examples
Embodiment 1
[0129] 4-isothiocyanato-2-trifluoromethylbenzonitrile, (1a)
[0130] 4-Amino-2-trifluoromethylbenzonitrile (2.23 g, 12 mmol) was added dropwise to a well stirred solution of thiophosgene (1 ml, 13 mmol) in water (22 ml) over 15 minutes at room temperature. in a homogeneous mixture. Stir for an additional 1 hour. The reaction mixture was extracted with chloroform (3 x 15ml). The combined organic phases were dried over magnesium sulfate and evaporated to dryness under reduced pressure to give the desired product, 4-isothiocyanato-2-trifluoromethylbenzonitrile, (1a), as a brown solid , which was used directly in the next step (2.72 g, 11.9 mmol, 99%).
Embodiment 2
[0132] 2-1).(4-Aminophenyl)carbamate tert-butyl ester, (2a)
[0133] An aqueous solution of potassium carbonate (1.52 g, 11 mmol in 5 ml water) was added to a solution of 1,4-phenylenediamine (3.24 g, 30 mmol) in THF (30 ml) and DMF (10 ml). To this mixture was added dropwise di-tert-butyl dicarbonate (Boc 2 O) (2.18 g, 10 mmol), added dropwise over 0.5 h. The reaction mixture was stirred at room temperature for 4 hours. The mixture was then poured into cold water (40ml) and extracted with chloroform (3 x 50ml). The combined organic phases were dried over magnesium sulfate and concentrated to give a brown residue, which was purified by flash chromatography (dichloromethane / acetone, 4:1) to give tert-butyl (4-aminophenyl)carbamate, (2a ), as a yellow solid (1.98g, 9.5mmol, 95%) (based on Boc 2 O yield).
[0134] 2-2). Tert-butyl {4-[(1-cyano-1-methylethyl)amino]phenyl}carbamate, 2b
[0135] 2a (0.83g, 4mmol), acetone cyanohydrin (acetone cyanohydrin, 2-methyl-2-hydroxypro...
Embodiment 3
[0147] 3-1).2-(4-hydroxyphenylamino)-2-methylpropionitrile, 3a
[0148] 4-Aminophenol (1.09g, 10mmol), acetone cyanohydrin (10ml) and MgSO4 (2g) were heated to 80°C and stirred for 4 hours. After concentrating the mixture under vacuum, compound 3a was crystallized from water (20ml). The solid was filtered off and dried to give 2-(4-hydroxyphenylamino)-2-methylpropionitrile, 3a (1.69 g, 9.6 mmol, 96%).
[0149] 3-2).4-[3-(4-Hydroxyphenyl)-5-imino-4,4-dimethyl-2-thioimidazolidin-1-yl]-2-trifluoromethyl Benzonitrile, 3b
[0150] Triethylamine (0.101 g, 1 mmol) was added to a solution of 1a (0.456 g, 2 mmol) and 3a (0.352 g, 2 mmol) in dry THF (5 ml). The reaction mixture was stirred at 0 °C for 48 hours, then concentrated to give a black residue, which was purified by flash chromatography (dichloromethane / acetone, 85:15) to give 4-[3-(4-hydroxyphenyl )-5-imino-4,4-dimethyl-2-thioimidazolidin-1-yl]-2-trifluoromethylbenzonitrile, 3b (0.274 g, 0.68 mmol, 34%).
[0151] 3-3).4-[...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com