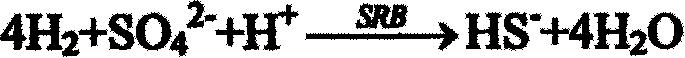Technique for processing low concentration heavy metal sulfate liquor with microorganism method
A technology of low-concentration heavy metals and microbial methods, which is applied in the field of wastewater treatment and low-concentration valuable metals, and can solve the problems that do not involve the separation and recovery of heavy metals and the concentration of heavy metal ions is not too high
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
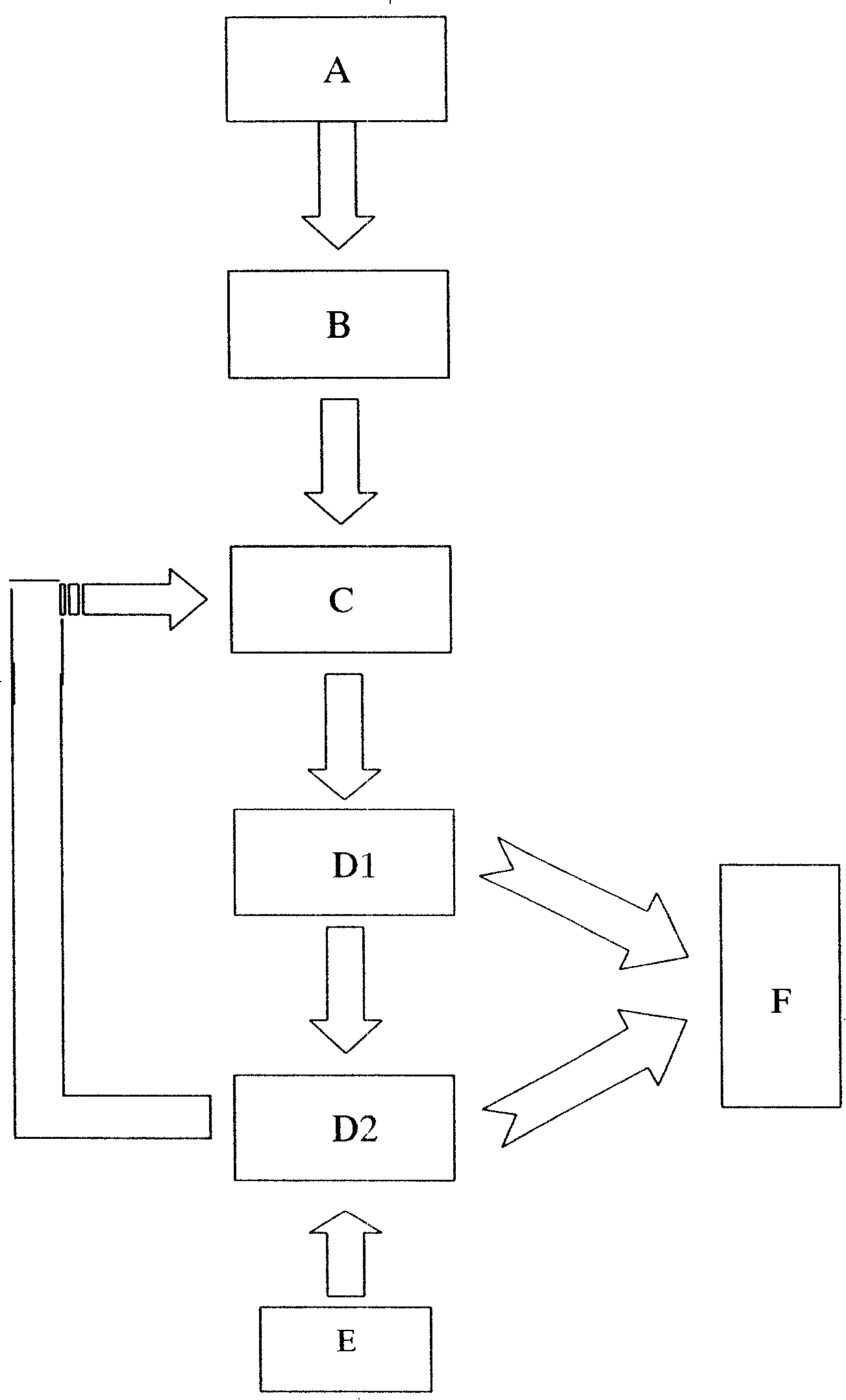
Image
Examples
Embodiment 1
[0024] The concentrations of metal ions in the biometallurgical leaching solution (pentlandite leaching solution) to be treated are respectively:
[0025] Fe 3+ : 5g / L, Ni 2+ : 2g / L, Cu 2+ : 0.5g / L, Mg 2+ : 20g / L
[0026] When the two units of acidogenic fermentation and sulfate-reducing bacteria-producing hydrogen sulfide are successfully started and run stably, and the total sulfide production is continuously stable above about 300mg / L, the outlet liquid in the reactor is poured into the precipitation reactor . The sulfate-reducing bacteria-producing hydrogen sulfide reactor is filled with ceramic rings as immobilized carriers. Cu 2+ The sulfide precipitate is formed first, and it is almost completely precipitated when the pH value is 1-3. The Cu finally detected in the treated leachate 2+ The content is less than 5mg; adjust the pH value, Fe 3+ and Ni 2+ When the pH value is 2-8, it gradually transforms into sulfide precipitation, and the final detected Fe in the s...
Embodiment 2
[0028] The concentrations of metal ions in the biometallurgical leaching solution (pentlandite leaching solution) to be treated are respectively:
[0029] Fe 3+ : 5g / L, Ni 2+ : 2g / L, Cu 2+ : 0.5g / L, Mg 2+ : 20g / L
[0030] After the two units of acidogenic fermentation and sulfate-reducing bacteria producing hydrogen sulfide are successfully started and run stably, and the total sulfide production is continuously stable above about 300 mg / L, the outlet liquid in the reactor is poured into the precipitation reactor . The sulfate-reducing bacteria-producing hydrogen sulfide reactor is filled with sponge as an immobilized carrier. Cu 2+ The sulfide precipitate is formed first, and it is almost completely precipitated when the pH value is 1-3. The Cu finally detected in the treated leachate 2+ The content is less than 2mg; adjust the pH value, Fe 3+ and Ni 2+ When the pH value is 2-8, it gradually transforms into sulfide precipitation, and the final detected Fe in the solu...
Embodiment 3
[0032] The concentrations of metal ions in the biometallurgical leaching solution (copper sulfide ore leaching tailing liquid) to be treated are respectively:
[0033] Fe 3+ : 5g / L, Cu 2+ : 10g / L
[0034] The specific implementation method is the same as embodiment 1, Cu 2+ First, sulfide precipitates are formed, which are almost completely precipitated when the pH value is 1-3, and the Cu finally detected in the treated leachate 2+ The content is less than 50mg; adjust the pH value, Fe 3+ When the pH value is 2-9, it gradually transforms into sulfide precipitation, and the final detected Fe in the leachate after precipitation 3+ The content is less than 12mg, and the recovery rate of valuable metals is high, copper is 99%, and iron is 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com