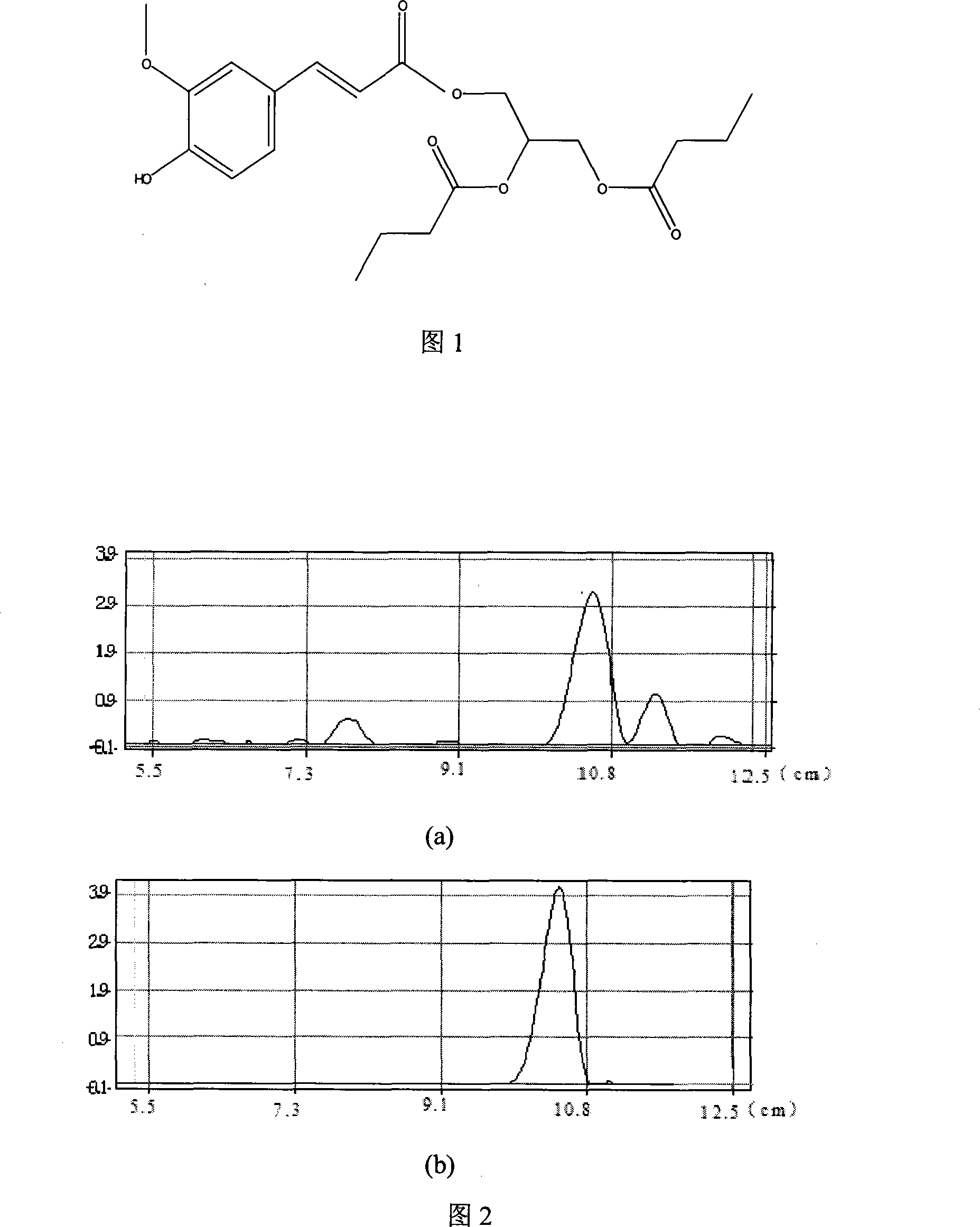
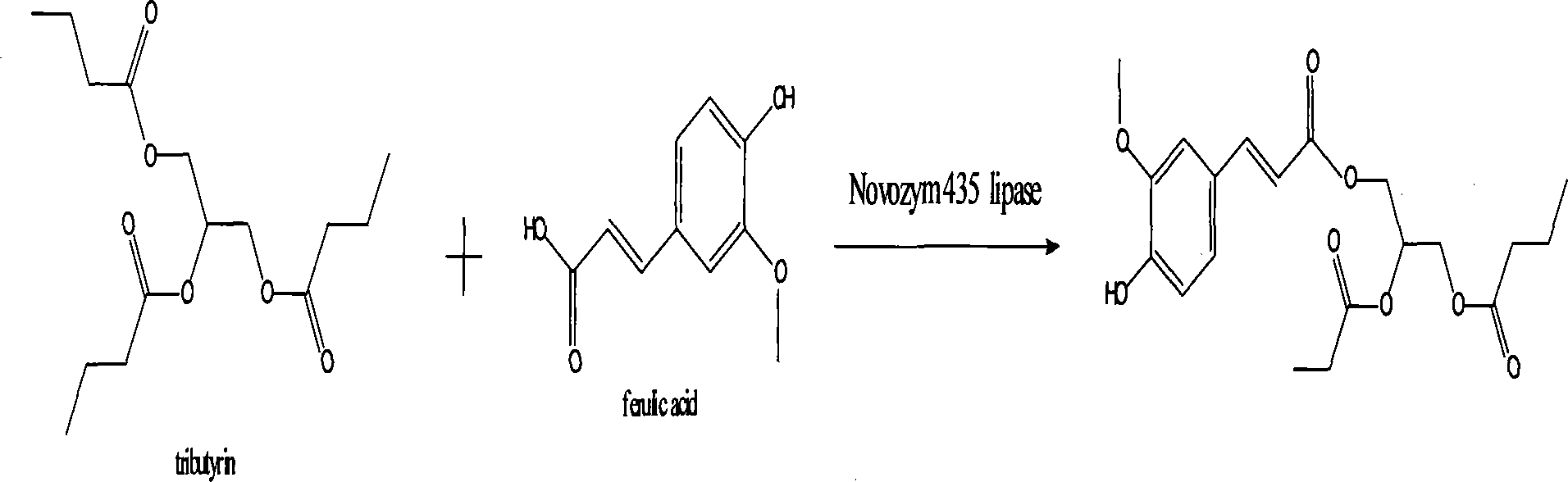
Method for synthesizing antioxidant feruloylated dibutyrated acylglycerol accelerated by solvent-free system enzyme
A technology of enzymatic synthesis of dibutyrin, which is applied in food preservation, food science, application, etc., can solve the problems of high recovery and recycling costs, flammable and volatile, and high toxicity, so as to reduce pollution and facilitate The effect of industrialization and fewer steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Substrate ferulic acid hydrophobization
[0028] 5g of ferulic acid and 50mL of absolute ethanol were catalyzed by 3mL of thionyl chloride, heated under reflux at 80°C and continuously stirred for 5h, and the reaction solution was rotatively evaporated to remove the solvent to obtain a light yellow oily substance, which was dissolved in a small amount of ether and washed with saturated bicarbonate The sodium solution was extracted for 3 times, and then the ether was removed by rotary evaporation, and the obtained liquid substance was allowed to stand still until a large amount of white needle-like crystals were precipitated to obtain ethyl ferulate.
[0029] (2) column chromatography purification of ethyl ferulate
[0030] Purification conditions: Packing: Take 25-30g of silica gel G, and use n-hexane as solvent to wet-pack the column, and control the flow rate to 1 drop / s. Chromatographic conditions: using the method of elution in stages, the ratio of the eluent i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com