Molecules and chimeric molecules thereof
A technology of chimeric molecules and compositions, applied in the direction of cytokines/lymphokines/interferons, hybrid peptides, cytokines/lymphokines/interferon receptors, etc., can solve inapplicable clinical applications, pollution, hazards Porting applications and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
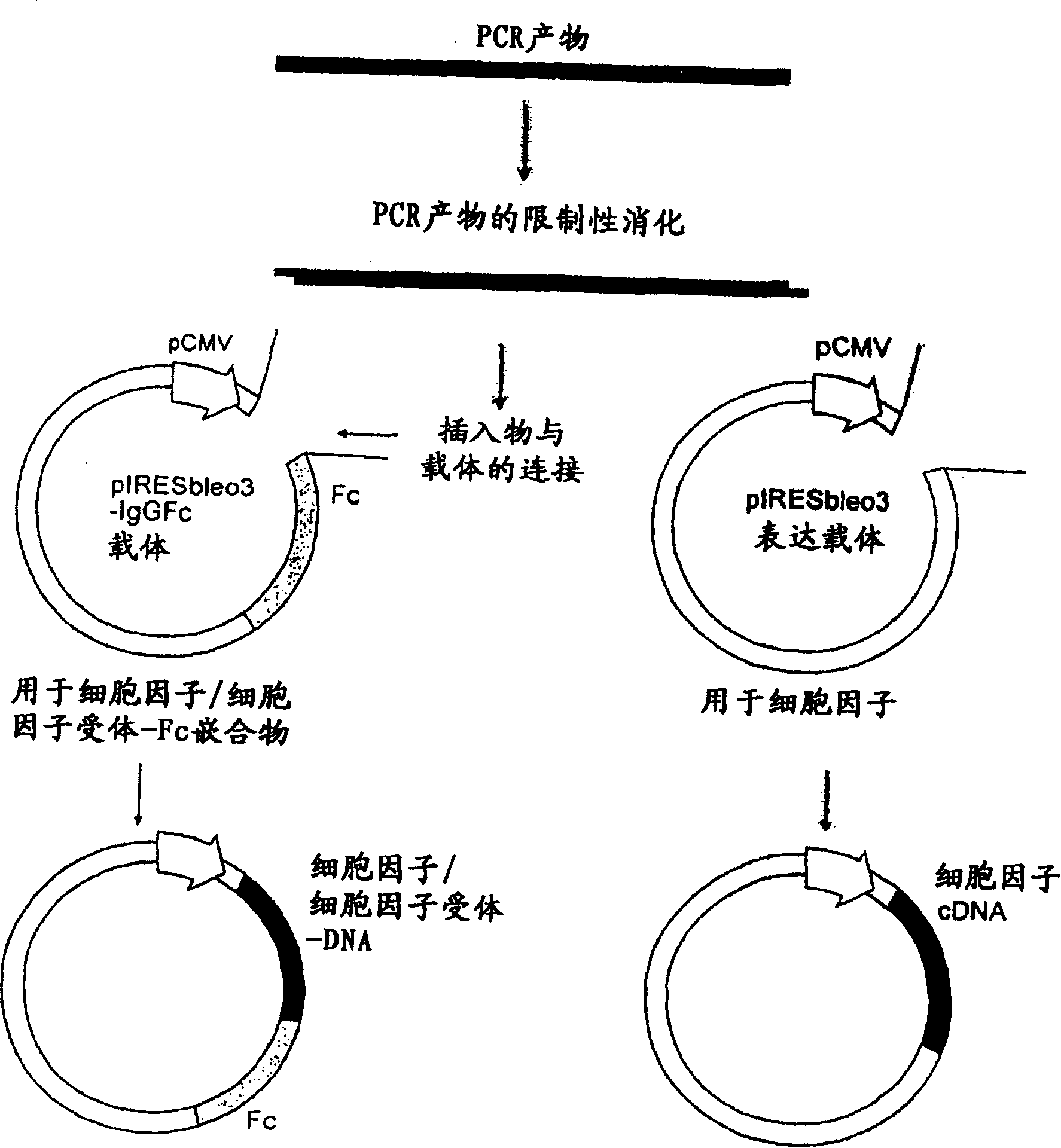
[1342] Preparation of vector-Fc construct
[1343] (a)pIRESbleo3-Fc
[1344] The DNA sequence encoding the Fc domain of human IgG1 was amplified by polymerase chain reaction (PCR), from the EST cDNA library (Clone ID 6277773, Invitrogen), using restriction enzymes BamH1 and BstX1 sites incorporated respectively Forward primer (SEQ ID NO: 21) and reverse primer (SEQ ID NO: 22). The amplicon was cloned into the corresponding restriction site in pIRESbleo3 (Cat. No. 6989-1, BD Biosciences) to prepare the construct pIRESbleo3-Fc. pIRESbleo3-Fc was digested with BamH1 and BstX1 to release an insert of the desired size of 780bp, as determined by gel electrophoresis.
[1345] (b) Preparation of DNA construct expressing protein or protein-Fc
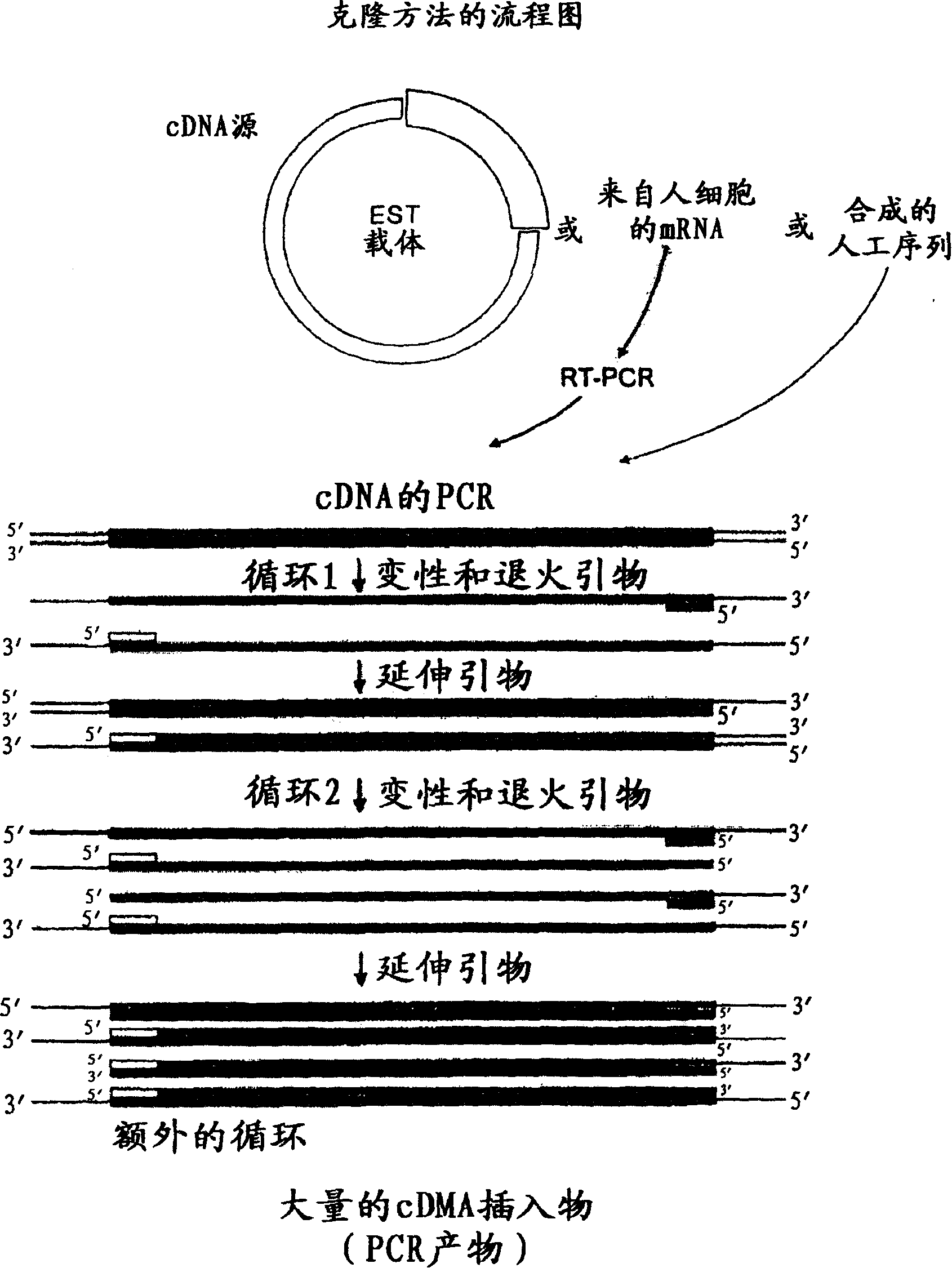
[1346] The DNA sequence encoding the protein or its extracellular domain was amplified by PCR, from the EST cDNA library, using forward and reverse primers with restriction enzyme cleavage sites introduced according to Table 8. After amplification, ...
Embodiment 2
[1356] (a) Preparation, separation and purification of TNF-α of the present invention
[1357] (i) Preparation of TNF-α of the present invention
[1358] On day 0, at 5 500cm 2 Spread 3×10 on the tissue culture dish (Corning) 7 Cells of a transformed human embryonic kidney cell line, such as HEK 293, HEK293cl8, HEK 293T, 293CEN4, HEK 239F, HEK 293E, HEK 293FT, AD-293 (Stratagene) or 293A (Invitrogen). To each culture dish, add 90ml Dulbecco's Modified Eagle's Medium / Ham Nutritional Complex F12 (DMEM / F12) (JRH Biosciences) to the cells, and add 10% (v / v) heat-inactivated fetal calf serum (FCS, JRH Biosciences) to the medium ), 4mM L-glutamine (Amresco), 10mM HEPES (Sigma), and 1% (v / v) penicillin-streptomycin (penicillin G 5000U / ml, streptomycin sulfate 5mg / ml) (JRHBiosciences). Petri dish at 37°C and 5% CO 2 Incubate overnight under the appropriate conditions.
[1359] On day 1, transfection was performed with calcium phosphate. Before transfection, the medium in each plate was re...
Embodiment 3
[1465] (a) Characterization of TNF-α of the present invention
[1466] (i) Two-dimensional polyacrylamide electrophoresis
[1467] Example 2(a) The collected sample was passed through a dialysis or desalting column (Pharmacia HR 10 / 10 Fast Desalting Column) to replace its buffer with repurified (18 MOhm) water, and dried with a SpeedVac concentrator. Alternatively, the collected samples can be precipitated with TCA or acetone using a known method. The sample was dissolved in 240μl of MSD buffer (5M urea, 2M thiourea, 65mM DTT, 2% (w / v) CHAPS, 2% (w / v) sultaine 3-10, 0.2% (v / v) Ampholyte carrier, 40mM Tris, 0.002% (w / v) bromophenol blue, water), and centrifuged at 15000g for 8 minutes.
[1468] Isoelectric focusing (IEF) was performed with precast 11cm or precast 17cm gel pH 3-10 solid phase pH gradient I EF strips (BioRad). The IEF strips are rehydrated in the sample in the closed tube at room temperature for at least 6 hours. The IEF strip is placed in the focusing chamber and c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com