Novel formulation of pyridoxal 5'-phosphate and method of preparation
A technology of pyridoxal phosphate and preparations, which can be applied in the directions of pill delivery, pharmaceutical formulations, and medical preparations of non-active ingredients, and can solve problems such as low doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
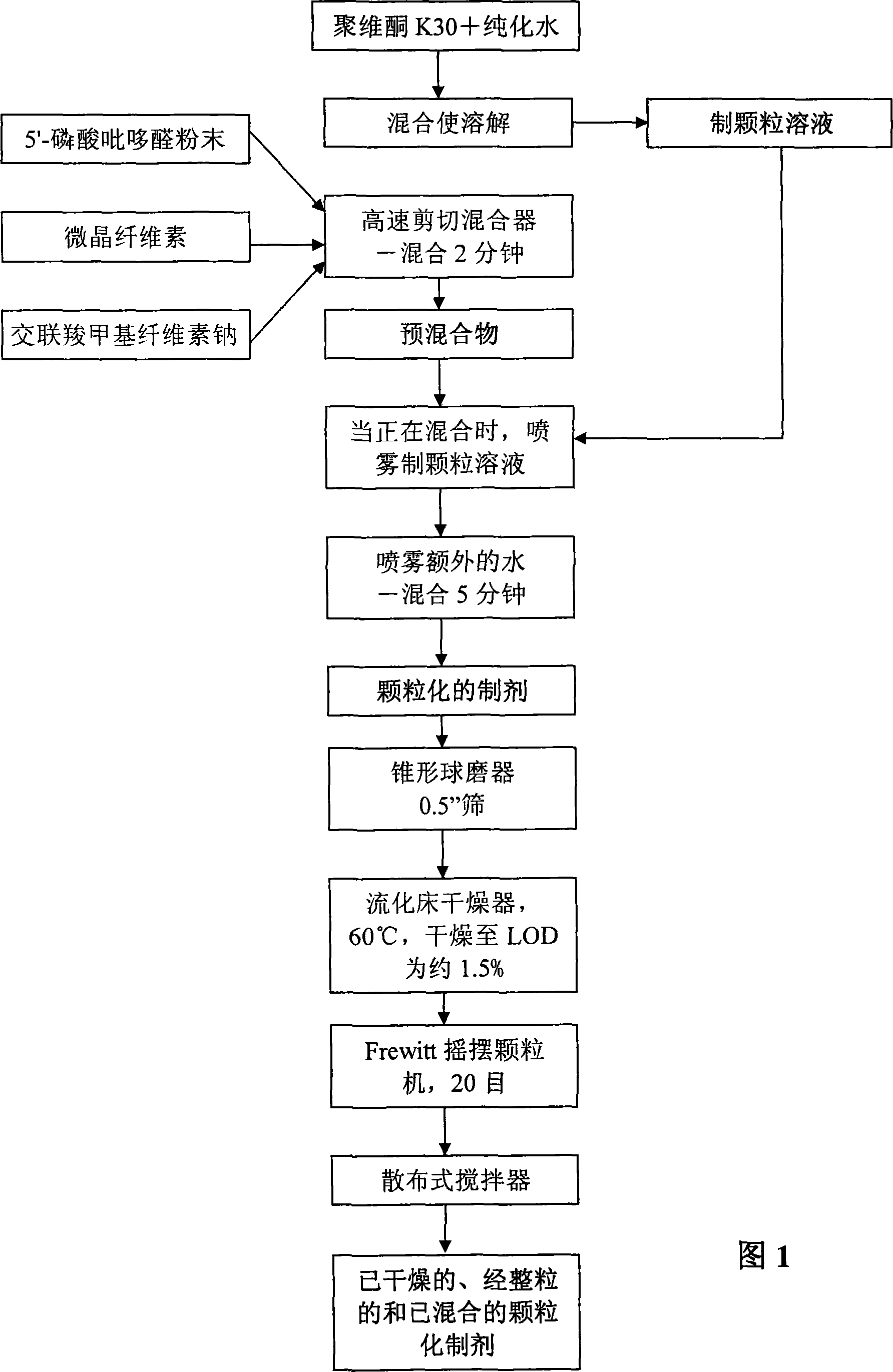
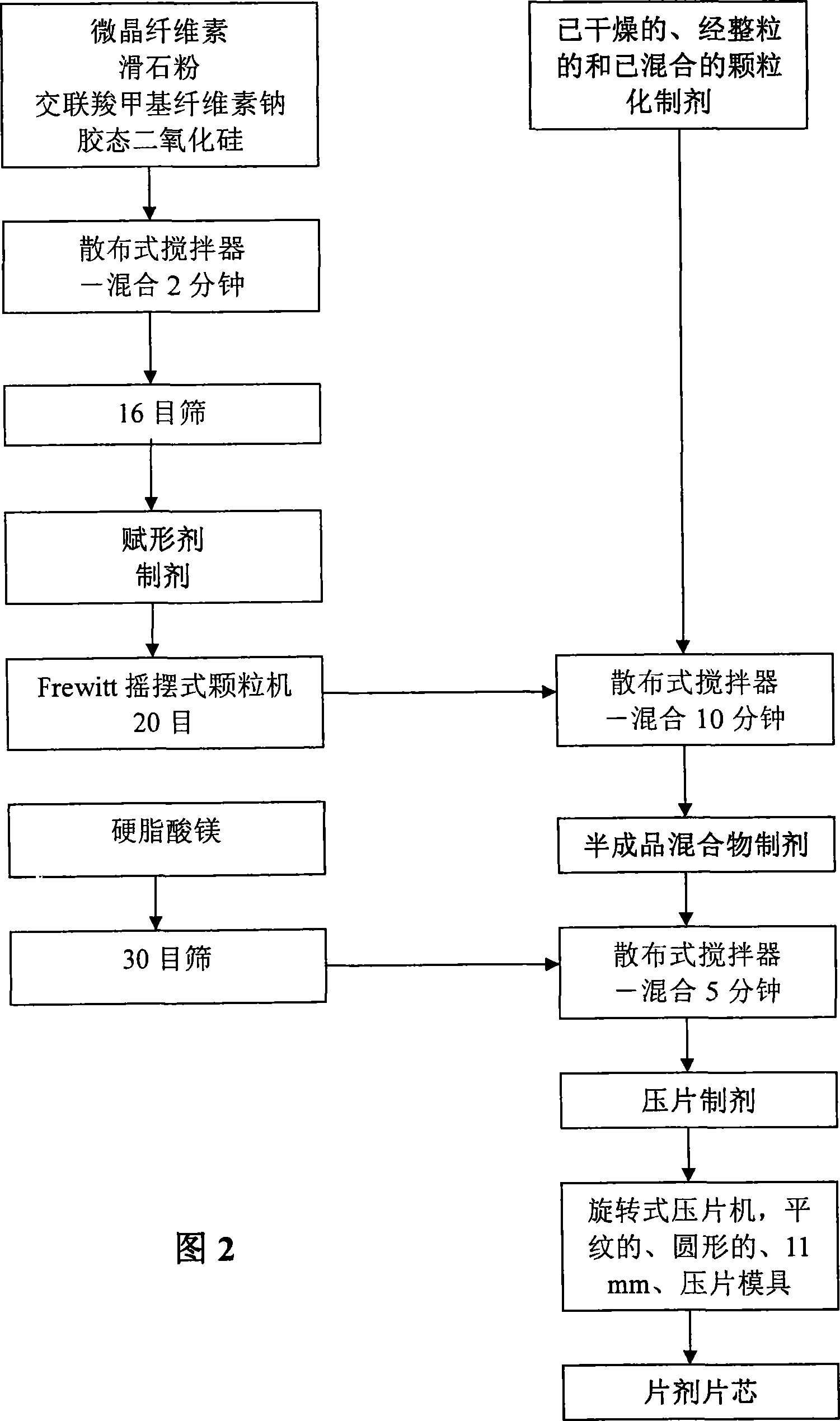
Image
Examples
Embodiment 1-5
[0118] Embodiment 1-5'-pyridoxal phosphate enteric-coated tablet formulation and preparation method thereof
[0119] Table 1 lists the ingredients and relative amounts (265 mg per tablet) of pyridoxal 5'-phosphate enteric-coated tablet preparations. As shown in Table 1, 20,000 tablets were produced in one batch. The batch size can be scaled up or down by proportionally increasing or decreasing the relative amounts.
[0120] Table 1: Pyridoxal 5′-phosphate enteric-coated tablet formulations
[0121] Element
%
mg / tablet
g / batch
Granulation stage
Pyridoxal 5′-phosphate
66.3
265
5300
Microcrystalline Cellulose (Avicel PH102)
11.9
47.5
950
2.0
8
160
Povidone (K-30)
4.7
18.75
375
Subtotal:
84.8
339.25
6785
Purified water (for PVP granule solution)
Other purified water (fo...
Embodiment 3-5
[0138] Dissolution test of embodiment 3-5'-pyridoxal phosphate enteric-coated tablet
[0139] The dissolution characteristics of 250 mg pyridoxal 5'-phosphate enteric-coated tablets were determined by conventional test methods.
[0140] Disintegration time was determined in artificial gastric juice (without pepsin) and artificial intestinal juice (without pancreatin) using USP method .
[0141] In artificial gastric juice, the tablets remained intact after 1 hour. Complete tablet disintegration was observed between 5:46 and 14:52 minutes in artificial intestinal fluid.
[0142] Dissolution times were determined using USP and USP method B for enteric-coated tablets. The paddle speed of the dissolution apparatus was set at 100 rpm, and the sampling points were set at 30 and 45 minutes. The concentration of pyridoxal 5'-phosphate in the dissolution buffer was determined by LCMS.
[0143] Dissolution data for enteric coated tablets were observed to be within the following sp...
Embodiment 4-5
[0147] Safety, tolerance and pharmacokinetic test of embodiment 4-5'-pyridoxal phosphate enteric-coated tablets
[0148] A single-centre, phase 1, open-label trial was conducted to evaluate the safety, tolerability, and pharmacokinetics of pyridoxal-5'-phosphate (p5p) enteric-coated tablets.
[0149] Subjects - Each test group consisted of 6 subjects (3 males, 3 females) consisting of:
[0150] ●Male or female, smoker or non-smoker, age ≥18 and ≤55.
[0151] ●Able to agree to participate in the trial.
[0152] ●BMI≥19.0 and 2 .
[0153] Subjects who meet any of the following conditions are excluded from this trial:
[0154] ●With clinically significant disease within 4 weeks prior to administration of study drug.
[0155] ●Clinically significant surgical procedure within 4 weeks prior to administration of study drug.
[0156] ●Any clinically significant abnormality found during drug screening.
[0157] ●Any reason for exclusion of the subject from the trial in the opinio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com