Protein and peptide medicine carrier and its preparation method and application
A technology of protein peptides and drugs, applied in drug combinations, pharmaceutical formulas, peptide/protein components, etc., can solve rare problems, achieve the effect of reducing damage, reducing surface tension, and simple and feasible process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
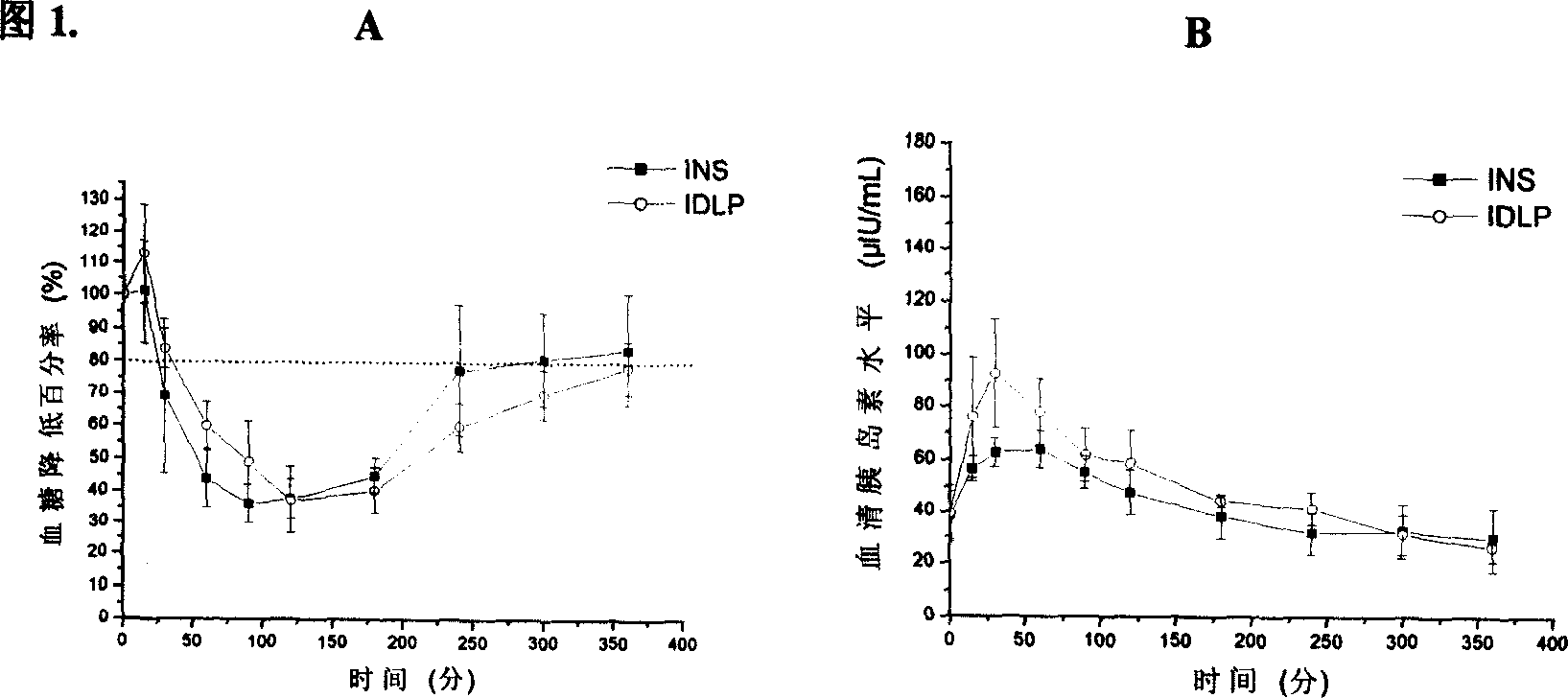
[0027] Take dipalmitoylphosphatidylcholine 13.5mg, phospholipid 6.8mg, palmitic acid 2.7mg, dissolve with chloroform and ethanol (1:1, V / V), remove the organic solvent by rotary evaporation, vacuum dry, add normal saline, 40- After hydration at 60°C for 2 hours, the artificial lung surfactant DLP was obtained by ultrasound. Insulin solution was added to the prepared DLP, and the ice bath was pulsed with ultrasound to obtain the INS-APS system-IDLP. Determination of the minimum surface tension of DLP and IDLP, see Table 1. 6 normal Wistar rats were fasted for 14 hours before the administration, without water. 4 IU / kg IDLP was given by tracheal infusion, blood was collected from the tail vein at 0, 15, 30, 60, 90, 120, 180, 240, 300, and 360 min, and centrifuged at 3000 rpm for 10 min. Glucose oxidase method was used to measure blood glucose level, and radioimmunoassay was used to measure serum insulin level, as shown in Figure 1. The relative pharmacological bioavailability ...
Embodiment example 2
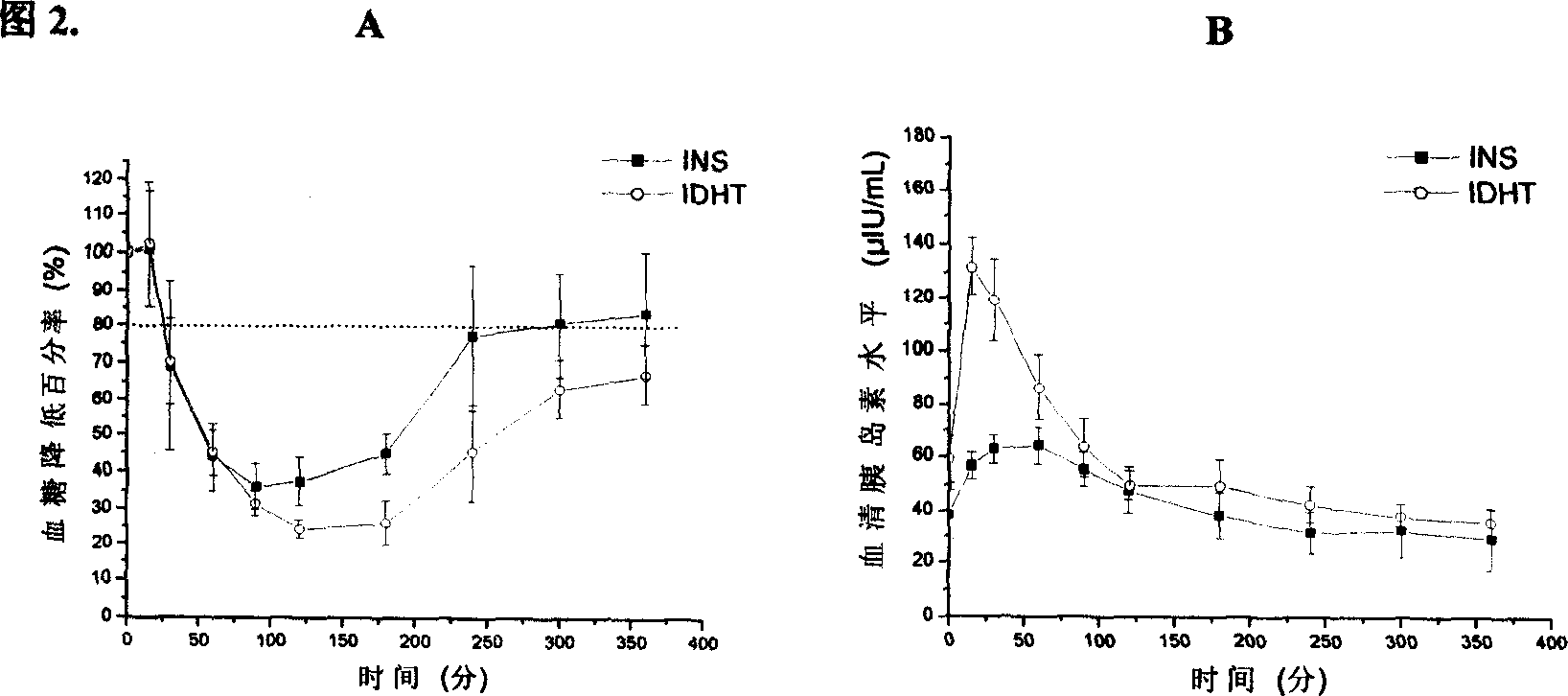
[0029] Take 13.5 mg of dipalmitoylphosphatidylcholine and 1.5 mg of cetyl alcohol, dissolve them in ethanol, remove the organic solvent by rotary evaporation, dry in vacuo, add tyloxapol normal saline solution, hydrate at 40-60°C for 2 hours, and obtain the product by ultrasonication Artificial lung surfactant DHT. Insulin solution was added to the prepared DHT, and the ice bath was pulsed with ultrasound to obtain the INS-APS system-IDHT. Determination of the minimum surface tension of DHT and IDHT, see Table 1. 6 normal Wistar rats were fasted for 14 hours before the administration, without water. 4IU / kgIDHT was given by tracheal infusion, blood was collected from the tail vein at 0, 15, 30, 60, 90, 120, 180, 240, 300, and 360 min, and centrifuged at 3000 rpm for 10 min. Glucose oxidase method was used to measure blood glucose level, and radioimmunoassay was used to measure serum insulin level, see Figure 2. The pharmacological relative bioavailability of IDHT reaches 38%...
Embodiment example 3
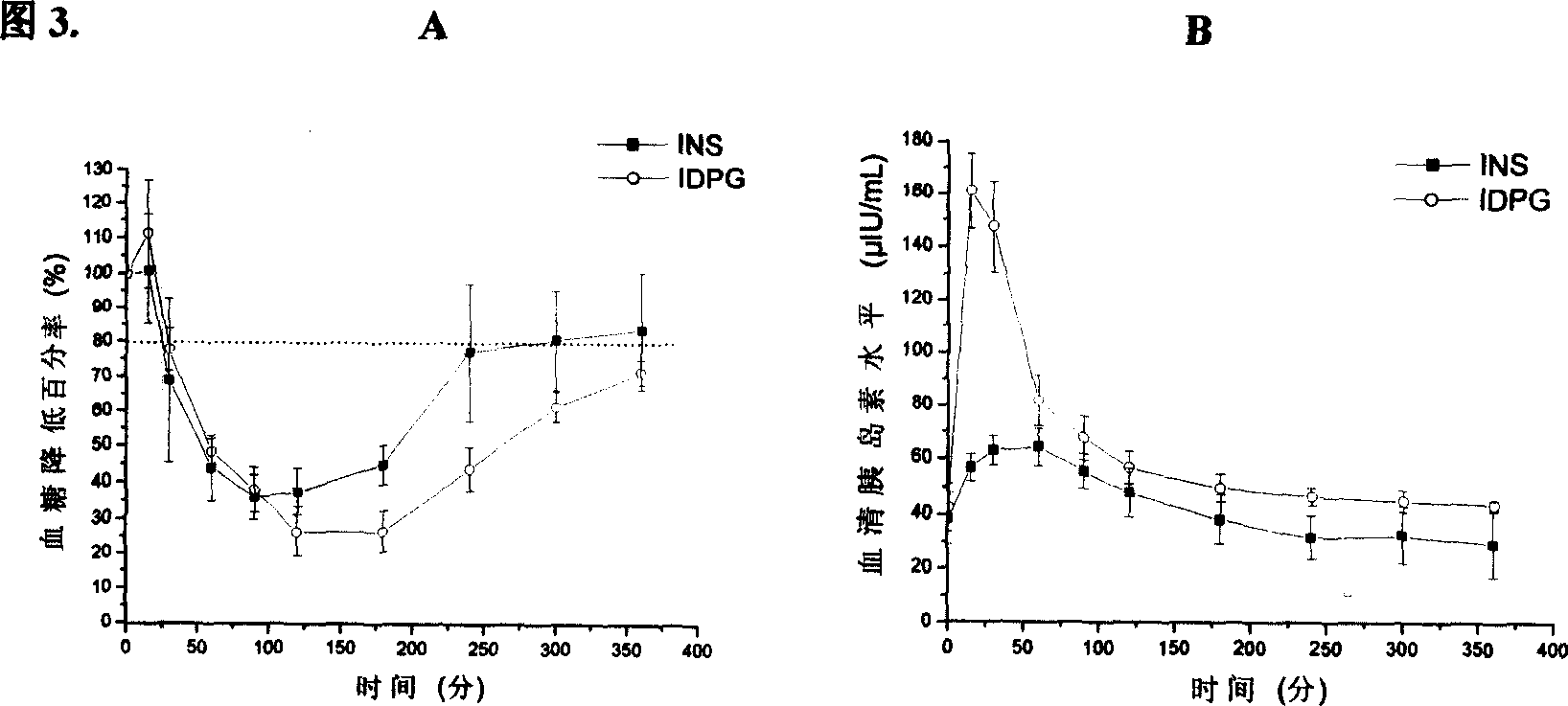
[0031]Take 13.5 mg of dipalmitoylphosphatidylcholine and 4.5 mg of phosphatidylglycerol, dissolve them in chloroform, remove the organic solvent by rotary evaporation, dry in vacuum, add normal saline, hydrate at 40-60°C for 2 hours, and obtain artificial lung surfactant by ultrasound DPG. Insulin solution was added to the prepared DPG, and the ice bath was pulsed with ultrasound to obtain the INS-APS system-IDPG. Determination of the minimum surface tension of DPG and IDPG, see Table 1. 6 normal Wistar rats were fasted for 14 hours before the administration, without water. 4 IU / kg IDPG was given by tracheal infusion, blood was collected from the tail vein at 0, 15, 30, 60, 90, 120, 180, 240, 300, and 360 min, and centrifuged at 3000 rpm for 10 min. Glucose oxidase method was used to measure blood glucose level, and radioimmunoassay was used to measure serum insulin level, see Figure 3. The pharmacological relative bioavailability of IDPG reaches 36%, which is 1.3 times tha...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com