Cationic elaioplast and its adenovirus composition, its preparing method and use
A technology of cationic liposomes and complexes, which is applied in the directions of liposome delivery, drug combination, pharmaceutical formulations, etc., can solve problems such as difficulty in obtaining in vivo curative effect, difficulty in determining liposomes, etc., and achieves improvement of raw materials and dosage, transfection The effect of improved efficiency and high encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 Cationic liposome formulation and preparation process of the present invention are preferred
[0045] Cationic liposomes were selected as the target liposome type, and after preliminary screening tests, dioleoylphosphatidylethanolamine (DOPE), N-[1-(2,3-dioleoyloxy)propyl]-N, N, N-trimethylammonium chloride (DOTMA), cholesterol (CHOL) as the basic components, and then optimize the formula.
[0046] The used raw material of the preparation method of liposome of the present invention and consumption are carried out optimal (seeing table 1) by the scope that initial screening obtains, process parameter and main technique are determined as follows:
[0047] According to Table 1, take 20-50 grams of DOPE, 20-50 grams of DOTMA, and 5-15 grams of cholesterol. The above raw materials are all solid; dissolve them with an organic solution of chloroform and methanol, and rotate them at a temperature not exceeding 40°C. Vacuum dry for 2 hours, add sterilized water, an...
Embodiment 2
[0052] The preparation of embodiment two cationic liposomes of the present invention
[0053] Dissolve 60mg of DOPE, 60mg of DOTMA and 6mg of cholesterol in 100ml of organic solvent of chloroform and methanol (volume ratio is 3:1, V / V), the temperature does not exceed 40 ℃, rotary evaporation, vacuum drying for 2 hours, add Sterilized water, under the protection of nitrogen, ultrasonically disrupted to obtain cationic liposomes. After aliquoting, store at 4°C-8°C for future use.
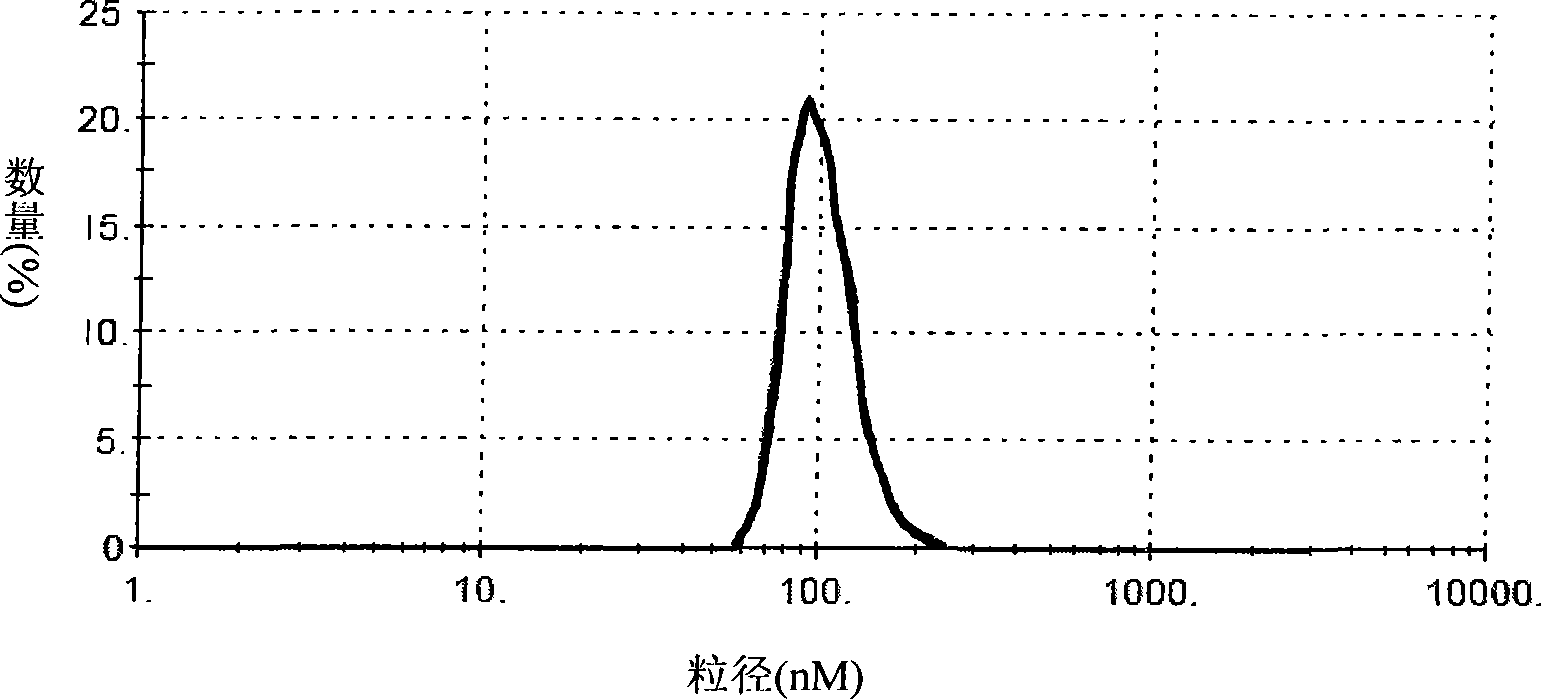
[0054] The transmission electron micrograph of the prepared cationic liposome is shown in figure 1 , it can be seen that the cationic liposome prepared by this method presents a multilayer vesicular structure with a particle size within a certain range. Prepared cationic liposomes are detected with a Malvern particle size analyzer, and the distribution diagram of the particle size curve is shown in figure 2 , it can be seen that the particle size of this cationic liposome is about 100nm, the dist...
Embodiment 3
[0055] Example 3 Construction and preparation of recombinant human endostatin adenovirus
[0056] The flow chart for the construction of recombinant human endostatin adenovirus is shown in Figure 4 (Refer to the published Chinese patent application for the construction process and the involved gene sequences, vectors, instruments and reagents: 200510021720 A recombinant human endostatin adenovirus and its preparation method and use), the process is as follows:
[0057] a. Constructing the coding sequence of human endostatin and the cloning vector pUC18 into pUC18-endo;
[0058] b. Using pUC18-endo as a template, use the PCR primers synthesized in step a to amplify the recombinant human endostatin gene containing IL-2 gene signal peptide.
[0059] c. Constructing the recombinant human endostatin gene obtained in step b into the pAdenoVator-CMV5 shuttle vector;
[0060] d. Co-transform Escherichia coli with the vector obtained in step c and the plasmid pAdenoVatorΔE1E3 contai...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com