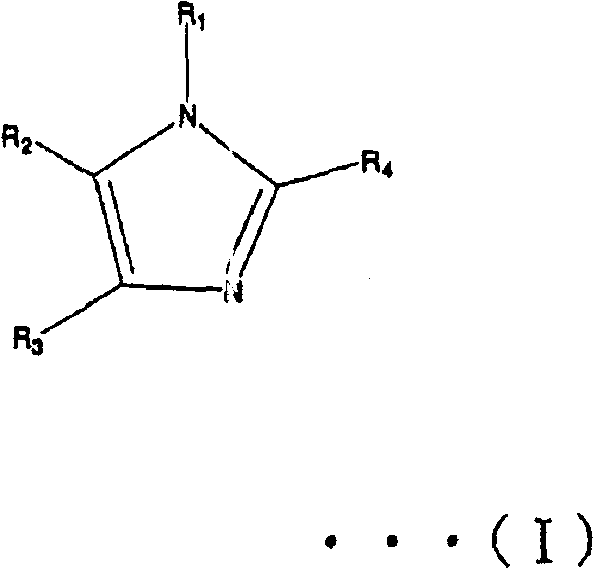
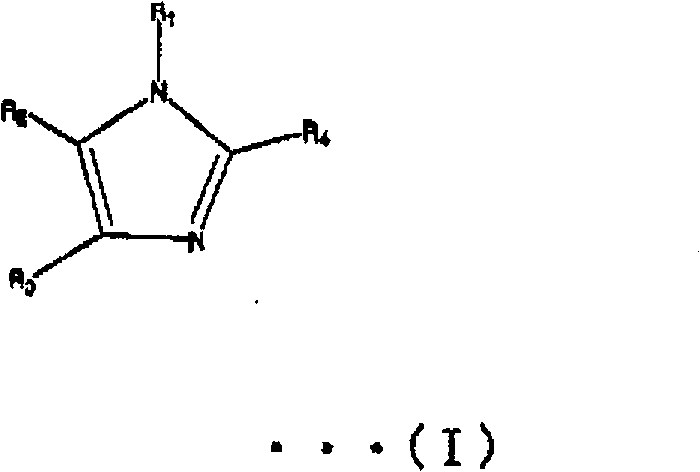
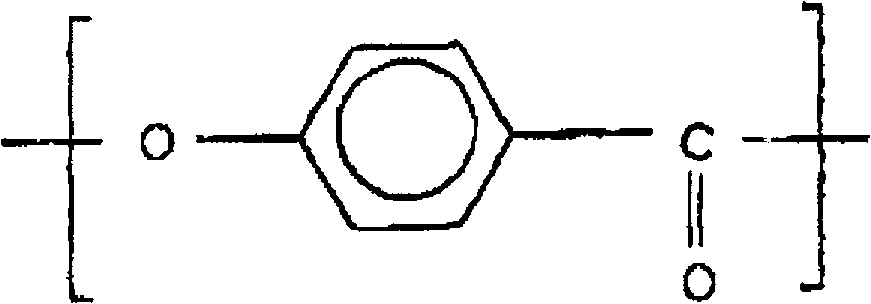
Liquid crystal polyester and its preparation method
A technology of liquid crystal polyester and transesterification, applied in liquid crystal materials, chemical instruments and methods, etc., can solve the problems of prolonged transesterification time and lack of impact strength of liquid crystal polyester, and achieve superior impact strength, superior heat resistance and tensile strength. The effect of tensile strength
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0092] Charge 1064 g (7.7 mol) p-hydroxybenzoic acid, 307 g (1.65 mol) 4,4'-dihydroxybiphenyl, 274.1 g (1.65 mol) terephthalic acid and 1235 g (12.1 mol) acetic anhydride. The reaction vessel was fully charged with nitrogen, then heated to 150° C. under nitrogen flow for 15 minutes, and the mixture was refluxed for 3 hours while maintaining the temperature.
[0093] Subsequently, 1.1 g of 1-methylimidazole was added, and then the mixture was heated to 320° C. for 2 hours and 50 minutes while distilling off by-product acetic acid and unreacted acetic anhydride. The recognition point of torque increase was considered to be complete, Remove the contents at this point. The resulting solid was cooled to room temperature and ground with a coarse grinder, then the mixture was heated from room temperature to 250°C over 1 hour and from 250°C to 335°C over 5 hours under nitrogen atmosphere and held at 335°C for 3 hours , to carry out the polymerization reaction in the solid layer. Th...
Embodiment 2~3, comparative Embodiment 1~3
[0096] The resin was obtained and its properties were measured in the same manner as in Example 1, except that the mixing ratio of the raw materials and the polymerization temperature in the solid layer were changed as shown in Table 1.
[0097] As for the obtained resin, the degree of crystallinity was measured with a polarizing microscope, and it was found to be a liquid crystalline polyester forming a melt phase having optical anisotropy similarly to Example 1.
Embodiment 4
[0099] Charge 911 g (6.6 mol) p-hydroxybenzoic acid, 409 g (2.2 mol) 4,4'-dihydroxybiphenyl, 274 g (1.65 mol) terephthalic acid, 91 g (0.55 mol) isophthalic acid and 1235 g (12.1 mol) acetic anhydride. The reaction vessel was fully charged with nitrogen, then heated to 150° C. under nitrogen flow for 15 minutes, and the mixture was refluxed for 3 hours while maintaining the temperature.
[0100] Subsequently, 1.1 g of 1-methylimidazole was added, and then the mixture was heated to 320° C. for 2 hours and 50 minutes while steaming the by-product acetic acid and unreacted acetic anhydride. remove the contents. The resulting solid was cooled to room temperature, ground with a coarse grinder, and the mixture was heated from room temperature to 250°C over 1 hour and from 250°C to 288°C over 5 hours under nitrogen atmosphere and kept at 288°C for 3 hours , to carry out the polymerization reaction in the solid layer.
[0101] Mixed glass (EFH-7501) manufactured by Central Glass wa...
PUM
| Property | Measurement | Unit |
|---|---|---|
| bending strength | aaaaa | aaaaa |
| bending strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com