Cyclic peptide containing arginine, glycine, asparagicacid-sequence and active target liposome
一种天冬氨酸、序列的技术,应用在主动靶向脂质体,治疗肝纤维化领域,达到良好抗纤维化疗效、分子量小、便于修饰的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1 prepares RGD polypeptide
[0037] Using the method reported in the literature (Schnolzer M, Alewood P, Jones A, Alewood D, KentSB. Int J Pept Protein Res.1992, 40 (3-4): 180-93), solid-phase synthesis of cysteine on a protein synthesizer -Glyn-ArgGlyAspSerProLys-Sch 2 CO-Leu-PAM) resin. Different from the literature, Boc-amino acid (2.2mmol) in N,N-dimethylformaldehyde containing condensing agent (HBTU 2.0mmol) and N,N-diisopropylethylamine (DIEA20%, v / v) Activated in amide (DMF) solution for 3 minutes, and then sequentially added to resin (0.25 mmol) to react for 10 minutes. The N-Boc protecting group was removed with trifluoroacetic acid (TFA); DMF and dichloromethane (DCM) were used to wash the resin throughout the synthesis. The side chain protecting groups of the amino acids used are: Arg (Tosyl), Asp (OcHxl), Cys (4MeBzl), Lys (2ClZ), Ser (Bzl). After the resin was synthesized, it was stirred at 0°C for 1 h in anhydrous hydrogen fluoride contain...
Embodiment 2
[0038] Embodiment 2 prepares liposome
[0039] Using rotary evaporation-film hydration-extrusion method, the liposomes were prepared through the following steps.
[0040] 1. Precisely weigh lecithin (EPC), cholesterol (Chol), monomethoxypolyethylene glycol-(mPEG) according to 2:1:0.1:0.02 (molar ratio) 2000 -DOPE), maleimide-polyethylene glycol-dipalmitoylphosphatidylethanolamine (MAL-PEG 3450 -DOPE), dissolved in chloroform, 40 ℃ water bath rotary evaporation to form a transparent film, evaporated to dry organic solvent. Phosphate buffered saline (PBS, pH 7.4, 22° C.) was added to fully hydrate. Use the Mini Extruder to repeatedly squeeze 15 times through the 100nm filter membrane to obtain uniform long-circulating liposomes (SSL). PEG accounts for 3.2 mol%.
[0041] Accurately weigh EPC, Chol, MAL-PEG according to 2:1:0.02 (molar ratio) 3450 -DOPE, dissolved in chloroform, evaporated in a water bath at 40°C to form a transparent film, and evaporated the organic solvent ...
Embodiment 3
[0048] Example 3 Using fluorescein and radioisotope tracer method to investigate the feasibility of artificially synthesized cyclic peptide containing RGD sequence as integrin receptor ligand
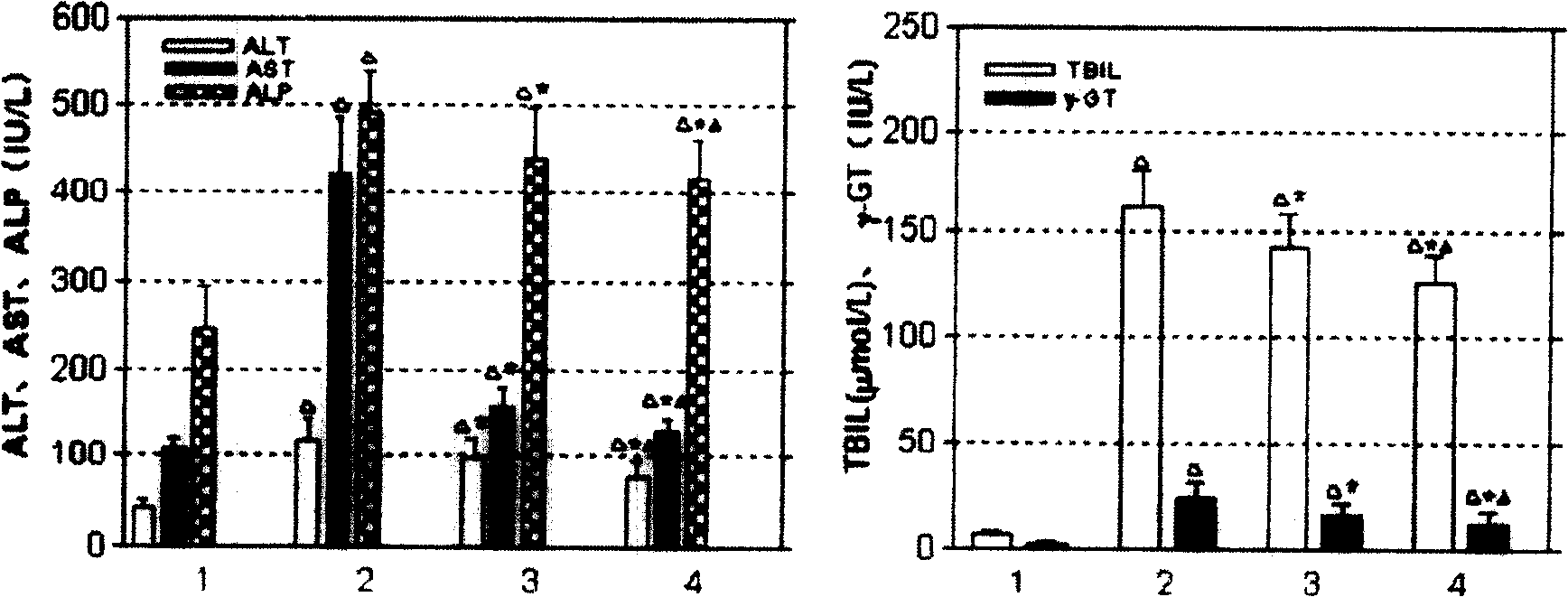
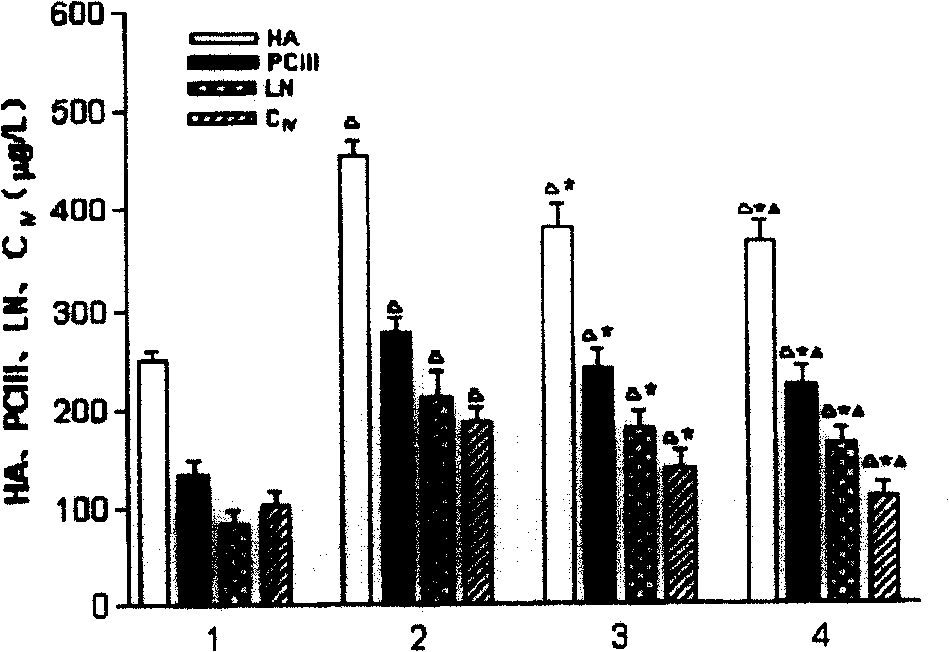
[0049] According to the properties of receptor-ligand binding, namely specificity, concentration and time dependence, competitive inhibition, fluorescein isothiocyanate (FITC)-labeled RGD cyclic peptide was co-incubated with HSCs. The results showed that the binding properties of RGD cyclic peptide to HSC conformed to the basic characteristics of receptor ligand. use 3 H-labeled RGD cyclic peptide was analyzed by radioligand Scatchard, and the equilibrium dissociation constant (Kd) and the number of binding sites (Bmax) on each cell were 7.05×10 -9 mmol / L and 6.79×10 5 .
[0050] Proceed as follows:
[0051] 1. Isolation of Rat HSCs
[0052] 2. Fluorescence tracer method to investigate the binding of activated HSC and cyclic peptide
[0053] HSCs were seeded on 6-well plates, and ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com