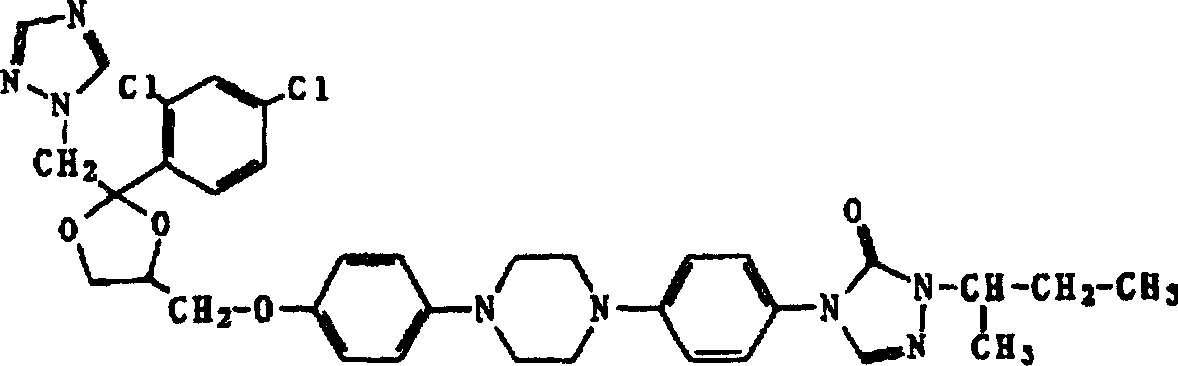
Itraconazole dripping pill and its prepn
A technology of itraconazole and azole dropping pills is applied in the directions of pill delivery, pharmaceutical formulations, medical preparations containing active ingredients, etc., and can solve the problems of unfavorable improvement of patients' ability to seek medical treatment, low bioavailability and high production cost, Achieve the effects of improved bioavailability, faster dissolution and absorption, and lower production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0036] Several specific examples are now used to further illustrate the preparation method of itraconazole dripping pills of the present invention.
[0037] [Selection of formula]
[0038] 1. Raw material: itraconazole
[0039] 2. Single substrate: selected from polyethylene glycol 1000 Polyethylene glycol 4000 Polyethylene glycol 6000 Polyethylene glycol 10000 Polyethylene glycol 20000 , Span 40, Polyoxy 40 stearate, poloxamer, sodium lauryl sulfate, stearic acid, sodium stearate, glycerin gelatin, shellac and other carriers;
[0040] 3. Combination base: in g or kg, by weight, select polyethylene glycol, polyoxy 40 stearate, sodium carboxymethyl starch, beta cyclodextrin, Tween and other carriers for combination test;
[0041] 3.1 Combination of two different substrates: take 1 part of polyoxyl 40 stearate or beta cyclodextrin or sodium carboxymethyl starch or Tween in units of g or kg by weight, and 1 part ~10 parts of polyethylene glycol in combination, where polyethylene glyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com